Chimeric Antigen Receptor (CAR) T-cell therapy has revolutionized the treatment of hematological malignancies, demonstrating significant clinical benefits. Despite its success, traditional autologous CAR T-cell therapies pose challenges such as systemic toxicity, complex manufacturing processes, and high costs, hindering their widespread adoption. In response to these challenges, Creative Biolabs presents CellRapeutics™ In Vivo Chimeric Antigen Receptor (CAR) Technology, a pioneering approach that aims to streamline the production of CAR T-cells, enhance safety, and broaden therapeutic applications.
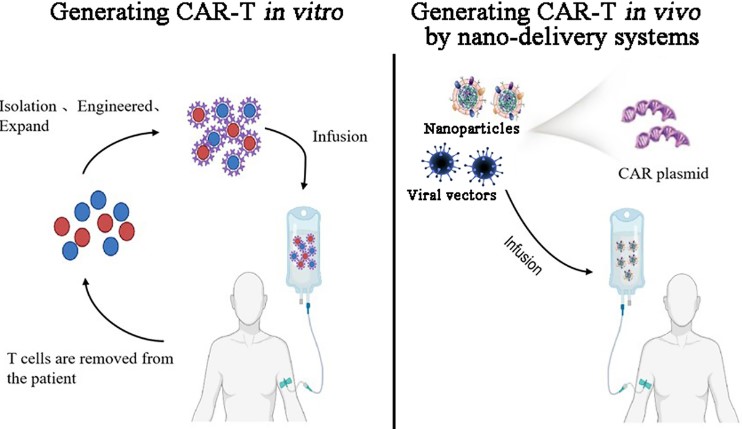 Fig.1 Schematic of CAR-T engineering ex vivo and in vivo.1
Fig.1 Schematic of CAR-T engineering ex vivo and in vivo.1
Leveraged by years of expertise in CART development, Creative Biolabs has developed the innovative CellRapeutics™ in vivo CAR platform. The in vivo CAR technology focuses on simplifying the T-cell manufacturing process by directly programming autologous T-cells in suit using viral-based vectors or nanocarriers loaded with CAR genes, and gene-editing tools. By eliminating the need for ex vivo T-cell manipulation, the process becomes less labor-intensive, more cost-effective, and accessible to a larger patient population. Our In Vivo Chimeric Antigen Receptor (CAR) Technologies include:
Simplified Manufacturing Process
The CellRapeutics™ platform eliminates the need for cumbersome ex vivo T-cell manipulation, drastically reducing production time and costs. This streamlined process enhances the feasibility of CAR T-cell therapy, making it more accessible to patients with rapidly progressing malignancies.
Enhanced Safety Profile
Programming T-cells in vivo, mitigates the systemic toxicities often associated with traditional CAR T-cell therapies, such as Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). This approach also avoids the safety concerns related to allogeneic CAR T-cells.
Broad Applicability
In vivo CAR technology presents a promise in extending CAR T-cell therapy beyond hematological malignancies to solid tumors and viral infections. The inclusion of gene-editing tools and cytokine genes aids in overcoming tumor immunosuppression and enhancing T-cell persistence and efficacy.
Creative Biolabs offers bespoke CAR design constructs and services tailored to specific cancer types and customers’ needs. Our experienced team utilizes cutting-edge techniques to develop CAR constructs with enhanced targeting and efficacy.
Our formulation experts specialize in developing nanoparticles optimized for in vivo gene delivery. These include lipid nanoparticles, nanocarriers, and polymer-based systems designed for stability and precise targeting.
Creative Biolabs provides comprehensive preclinical support, including in vivo efficacy testing, safety assessments, and regulatory guidance. Our integrated services ensure a seamless transition from preclinical research to clinical trials.
Q1: How does the nanoparticle-mediated delivery work?
A1: The nanoparticles are engineered to target specific T-cell markers, ensuring the precise delivery of CAR genes or gene-editing tools directly into T-cells within the body, thereby inducing the formation of functional CAR T-cells in situ.
Q2: What are the safety benefits of in vivo CAR T-cell programming?
A2: In vivo programming reduces systemic toxicities such as CRS and ICANS by enabling localized gene editing within the patient. It also avoids the complications associated with allogeneic CAR T-cells, such as graft-versus-host disease.
Q3: How does Creative Biolabs support clinical translation?
A3: Creative Biolabs offers a full suite of services from custom CAR design to nanoparticle formulation and regulatory support, ensuring that the transition from preclinical research to clinical application is smooth and efficient.
With our years of experience, Creative Biolabs continues to advance CellRapeutics™ in vivo chimeric antigen receptor (CAR) technology to meet the needs of CART development. Feel free to contact us for further communication.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION