Based on our research and development experience in TCR-T cell therapy, Creative Biolabs has developed a platform for non-viral vector delivery cell development in allogeneic TCR-T cell therapy. We are confident that our manufacturing platform will facilitate the efficient development and production of a variety of TCR-T therapies.
TCR delivery refers to the process of introducing engineered TCRs into T cells to enable them to recognize and attack cancer cells or other target cells expressing specific antigens. This process is a crucial component of TCR-T cell therapy, which aims to enhance the immune response against tumors.
These aspects of TCR delivery are critical for the successful implementation and effectiveness of TCR-T cell therapies:
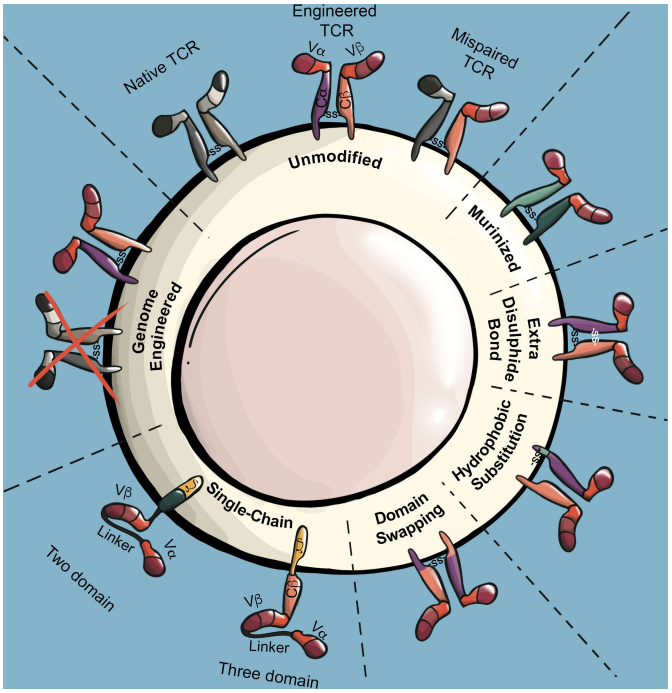 Fig.1 The modifications can enhance the performance and safety of the TCRs being delivered into T cells.1
Fig.1 The modifications can enhance the performance and safety of the TCRs being delivered into T cells.1
The aAPCs can be used to stimulate T cells in vitro, providing a platform for TCR discovery and expansion before the TCRs are delivered into patient-derived T cells.
The TCR genes are transferred into T cells using various methods. The most commonly used strategies for TCR gene transfer include viral vectors and electroporation techniques (non-viral methods). Lentiviral and retroviral vectors are commonly used to transduce T cells with TCR genes, allowing for stable expression of the engineered TCRs. Non-viral methods, like transposon systems, may reduce the risk of insertional mutagenesis.
| Viral vectors | |
|
Electroporation methods (Non-viral methods) |
|
Streamlining the manufacturing process to reduce complexity and cost, including the use of allogeneic (off-the-shelf) TCR-T products, can make therapies more accessible. Creative Biolabs has established a non-viral vector delivery platform that operates on a transposon/transposase system. The TCR-encoding DNA is encapsulated in a plasmid in this system. The plasmid, along with an mRNA sequence for a transposase enzyme, is introduced into T cells through electroporation. The transposase then facilitates the integration of the TCR sequence from the plasmid into the T cell's genome.
Our platform allows for larger cargo sizes, enabling the delivery of both TCRs and functional enhancements to T cells. Additionally, the process requires only a specific plasmid for each TCR product and a constant mRNA sequence, thereby simplifying the manufacturing process. Furthermore, it also provides a cost-effective solution, as the transposon system offers lower and faster manufacturing costs compared to lentiviral methods.
Please contact us and we would be pleased to help you accelerate your research through our non-viral vector delivery cell development services.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION