All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs offers various MICA CAR-T cell products, including CAR vectors, CAR mRNA, and CAR cells. These products are designed to enable researchers to develop and investigate the therapeutic potential of MICA CAR-T cells in cancer treatment.
MICA, or MHC class I polypeptide-related sequence A, is a protein that belongs to the major histocompatibility complex (MHC) family and is encoded by the MICA gene in humans. It is expressed on the surface of cells and plays a crucial role in immune responses. It acts as a ligand for the NKG2D receptor found on NK cells, CD8+ T cells, and some γδ T cells. The binding of MICA to NKG2D activates these immune cells and triggers targeted killing of the MICA-expressing cells, such as tumors or virus-infected cells. MICA overexpression has been associated with various diseases, including prostate cancer, breast cancer, and autoimmune disorders.
Associated Disease
Creative Biolabs offers comprehensive in vitro assays to assess the efficacy and safety of anti-MICA CAR-T cell therapy, including:
Our anti-MICA CAR-T expression tests typically include Western Blot (WB) and Flow Cytometry (FC) analyses. By combining WB and FC analyses, researchers can assess the expression levels and functionality of the engineered CAR-T cells, which is crucial for therapeutic efficacy.
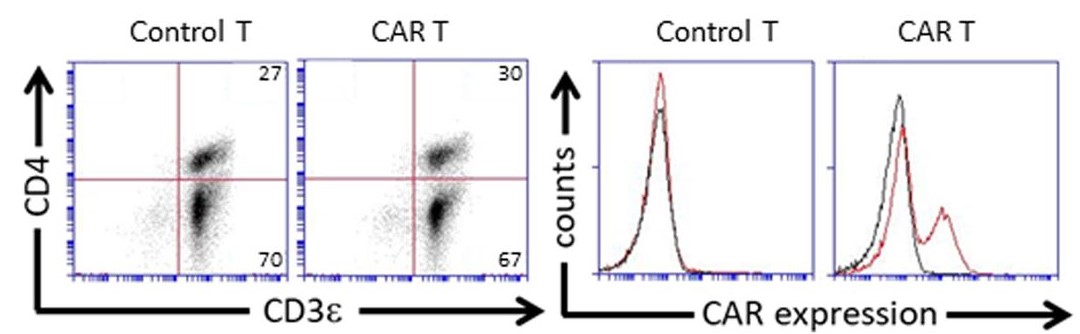
Fig.1 Flow analysis of the anti-MICA CAR expression.1
The proliferation test typically measures the rate of cell growth of CAR-T cells in vitro following exposure to target antigens. We provide common proliferation assays such as flow cytometry, cell counting, and proliferation assays using techniques like CFSE or Ki67 staining.
The cytokine release tests are used to determine the safety and efficacy of CAR T-cell therapies by examining the release of cytokines by the CAR-T cells when they coculture with the target cancer cells. At our lab, our skilled team has the ability to measure the levels of various cytokines released by CAR-T cells and ensures accurate and efficient results for all your CAR-T cytokine release testing needs. The levels of cytokines are measured using various assays, such as ELISA or flow cytometry.
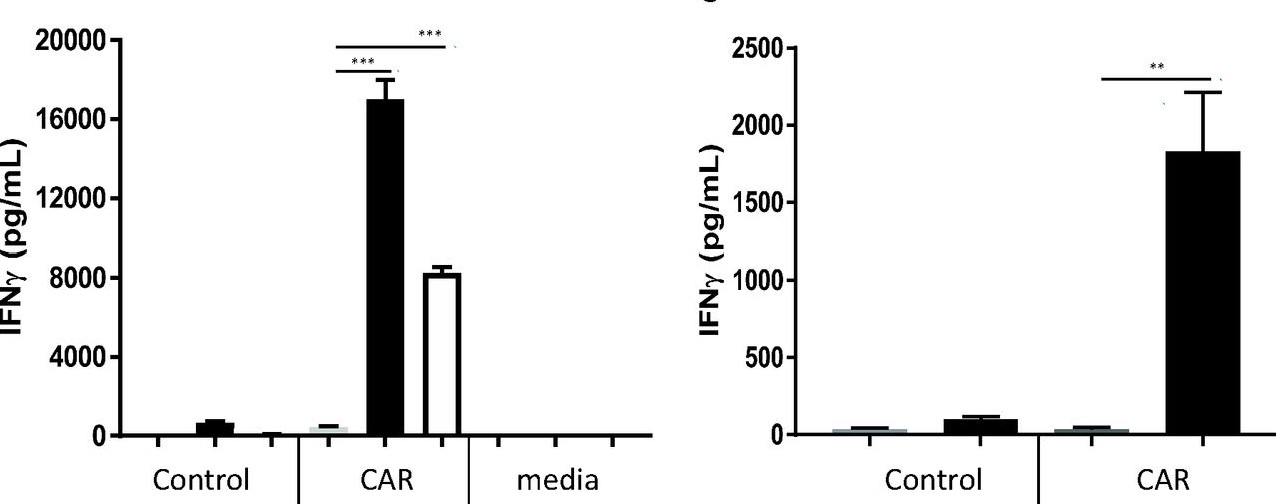
Fig.2 The measurement of IFN-γ production by ELISA.1
Our team offers a range of in vitro CAR-T cell cytotoxicity assays to meet the needs of different research projects. These assays are important for assessing the potency and efficacy of CAR-T cell therapies before they are used in clinical studies.
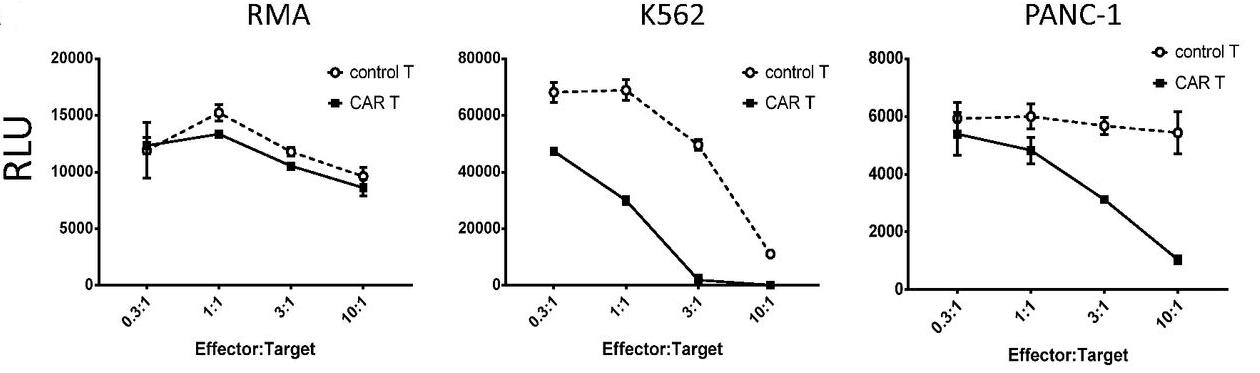
Fig.3 Cytotoxicity of anti-MICA CAR-T cells against MICA-positive tumor cells as measured by luciferase cytotoxicity assay.1
We provide comprehensive in vivo assays to evaluate the efficacy of anti-ERBB3 CAR-T cells in vivo. Our team of experts is highly experienced in performing in vivo assays and analyzing the resulting data using appropriate statistical methods, supporting researchers in determining the efficacy of Anti-GD2 CAR-T cells in suppressing tumor growth.
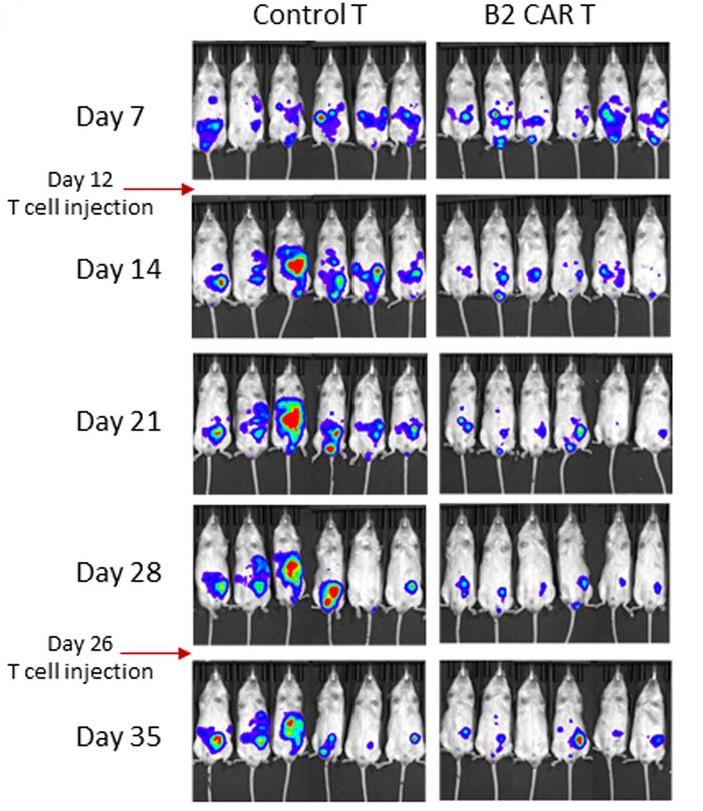
Fig.4 The anti-tumor activity of anti-MICA CAR-T cells in a PANC1 tumor model. The CAR-T cells inhibited PANC-1 progression.1
Reference
 Loading...
Loading...
| CAT | Product Name | Target Species | Antibody Clone | Antibody Host | Receptor Construction | Vector Type | Targeting Cell Type | CAR Vector Type | Inquiry & Datasheet |
| CAR-T-2-L407-BZ | MICA h(41BB-CD3ζ) CAR, [T] | Human | Human | scFv-41BB-CD3ζ | Lentiviral | T cell | |||
| CAR-T-2-L407-CZ | MICA h(b2c-CD3ζ) CAR, [T] | Human | Human | scFv-b2c-CD3ζ | Lentiviral | T cell | |||
| CAR-T-3-L407-2BZ | MICA h(CD28-41BB-CD3ζ) CAR, [T] | Human | Human | scFv-CD28-41BB-CD3ζ | Lentiviral | T cell | |||
| CAR-T-2-L407-2Z | MICA h(CD28-CD3ζ) CAR, [T] | Human | Human | scFv-CD28-CD3ζ | Lentiviral | T cell | |||
| CAR-T-2-L407-2G | MICA h(CD28-FcεRIγ) CAR, [T] | Human | Human | scFv-CD28-FcεRIγ | Lentiviral | T cell | |||
| XS-0822-YF645 | Anti-Human MICA (XW-645) h(41BB-CD3ζ) CAR IVT Plasmid, pCARIVT | Human | XW-645 | Human | scFv-41BB-CD3ζ | In Vitro Transcription (IVT) Vector | |||
| XS-0822-YF1565 | Anti-Human MICA (XW-645) h(CD28-CD3ζ) CAR IVT Plasmid, pCARIVT | Human | XW-645 | Human | scFv-CD28-CD3ζ | In Vitro Transcription (IVT) Vector | |||
| XS-0822-YF2485 | Anti-Human MICA (XW-645) h(CD28-41BB-CD3ζ) CAR IVT Plasmid, pCARIVT | Human | XW-645 | Human | scFv-CD28-41BB-CD3ζ | In Vitro Transcription (IVT) Vector | |||
| XS-1122-YF645 | Anti-MICA KIR CAR (scFv-KIR2DS2-DAP12, XW-645), pCDCAR1 | Human | XW-645 | Human | scFv-KIR2DS2 TM&ICD-2A-DAP12 | Lentiviral vector | T Cell | ||
| XS-1122-YF1565 | Anti-MICA TCR-ABR (scFv-TCRα, XW-645) CAR Plasmid, pCDCAR1 | Human | XW-645 | Human | scFv-TCRα | Lentiviral vector | T Cell | ||
| XS-1122-YF2485 | Anti-MICA TCR-ABR (scFv-TCRβ, XW-645) CAR Plasmid, pCDCAR1 | Human | XW-645 | Human | scFv-TCRβ | Lentiviral vector | T Cell | ||
| XS-1122-YF3405 | Anti-MICA TCR-ABR (scFv-CD3γ, XW-645) CAR Plasmid, pCDCAR1 | Human | XW-645 | Human | scFv-CD3γ | Lentiviral vector | T Cell | ||
| XS-1122-YF4325 | Anti-MICA TCR-ABR (scFv-CD3δ, XW-645) CAR Plasmid, pCDCAR1 | Human | XW-645 | Human | scFv-CD3δ | Lentiviral vector | T Cell | ||
| XS-1122-YF5245 | Anti-MICA TCR-ABR (scFv-CD3ε, XW-645) CAR Plasmid, pCDCAR1 | Human | XW-645 | Human | scFv-CD3ε | Lentiviral vector | T Cell | ||
| XS-1122-YF6165 | Anti-MICA (XW-645) h(CD28-CD3ζ) CAR, pAAV | Human | XW-645 | Human | scFv-CD28-CD3ζ | Adeno-associated viral (AAV) vector | T Cell | ||
| XS-1122-YF7085 | Anti-MICA (XW-645) h(41BB-CD3ζ) CAR, pAAV | Human | XW-645 | Human | scFv-41BB-CD3ζ | Adeno-associated viral (AAV) vector | T Cell | ||
| XS-1122-YF8005 | Anti-MICA (XW-645) h(CD28-41BB-CD3ζ) CAR, pAAV | Human | XW-645 | Human | scFv-CD28-41BB-CD3ζ | Adeno-associated viral (AAV) vector | T Cell | ||
| XS-0123-ZP832 | Anti-MICA (mAb2) h(scFv-CD3ε) TRuC, pCDTRC1 | Human | mAb2 | Human | scFv-CD3ε | Lentiviral vector | T cell | ||
| XS-0323-ZP832 | Anti-MICA (mAb2 scFv-CD28TM-CD79β) CBCR(CAR-B), pCDCAR1 | Human | mAb2 | Human | scFv-CD28TM-CD79β | Lentiviral vector | T cell | CAR-B Vector | |
| XS-0923-LX118 | Anti-hMICA (CD28-41BB-CD3ζ) CAR-duplex CTLA4 pCDCAR1 Vector | Human | X9X-87 | scFv-CD28-41BB-CD3ζ-duplex CTLA4 | Lentiviral vector | T cell | CAR-T |
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION