Creative Biolabs provides a broad-spectrum tumor antigen dendritic cell (DC) development service that employs advanced DC engineering and comprehensive whole tumor lysate pulsing using both autologous and allogeneic sources to generate potent, broad-spectrum anti-tumor immunity and streamline the development of effective and broadly applicable immunotherapies.
Cancer's intricate heterogeneity and its proficiency in evading immune surveillance demand innovative therapeutic strategies. Dendritic cells (DCs), as crucial regulators of adaptive immunity, possess a unique ability to present a diverse range of tumor antigens. This includes tumor-associated antigens (TAAs), which are commonly expressed by cancer cells, and patient-specific neoantigens (NeoAgs), which arise from somatic mutations within tumors. By presenting these antigens, DCs can effectively prime and activate robust anti-tumor T-cell responses, making them a promising avenue for cancer immunotherapy.
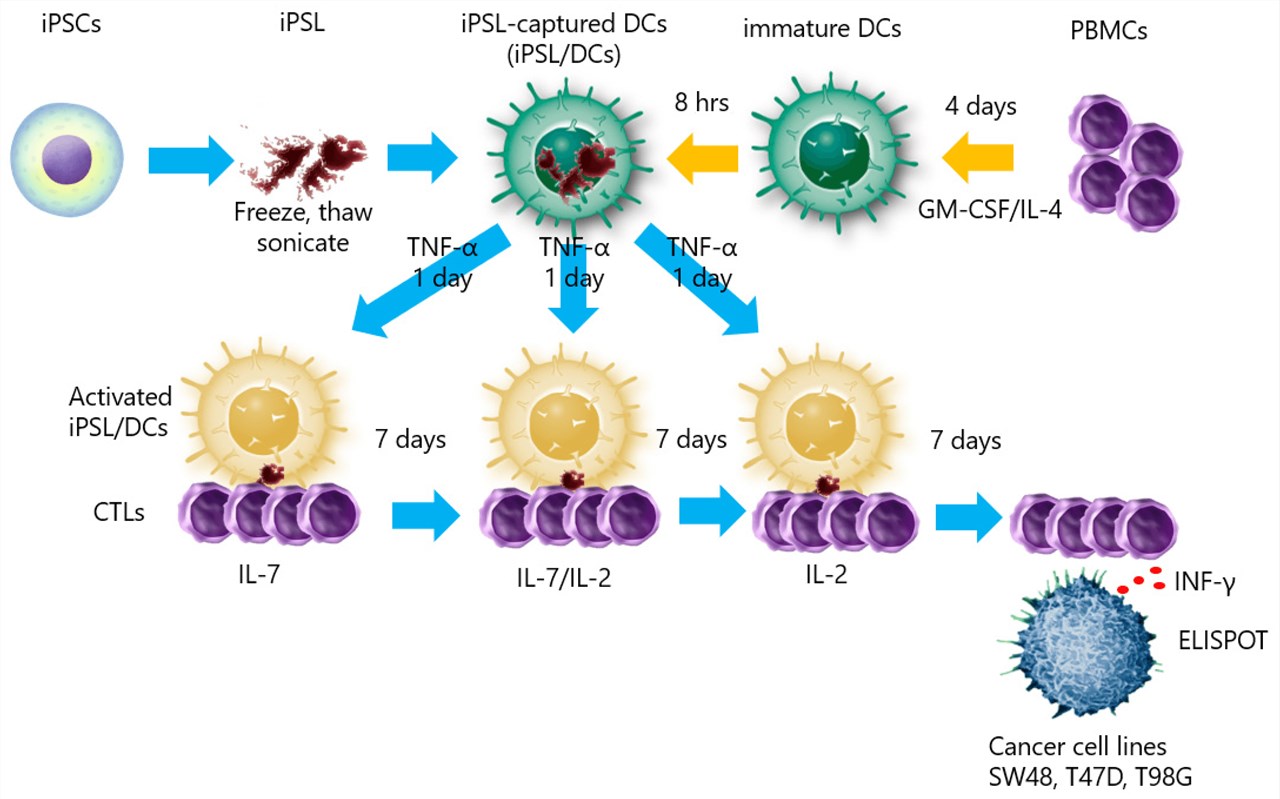 Fig.1 Dendritic cells loaded with induced-pluripotent stem cells lysate for inducing cytotoxic T lymphocytes.1
Fig.1 Dendritic cells loaded with induced-pluripotent stem cells lysate for inducing cytotoxic T lymphocytes.1
Creative Biolabs delivers specialized solutions designed to accelerate your cancer immunotherapy initiatives. Our service provides customized DC vaccine development protocols tailored to your unique research or clinical objectives. We ensure the production of highly functional, antigen-loaded DCs, whether derived from autologous patient samples or robust allogeneic sources. Clients benefit from our comprehensive preclinical validation support, including advanced immunogenicity assays, to characterize the immune responses elicited by the developed DCs.
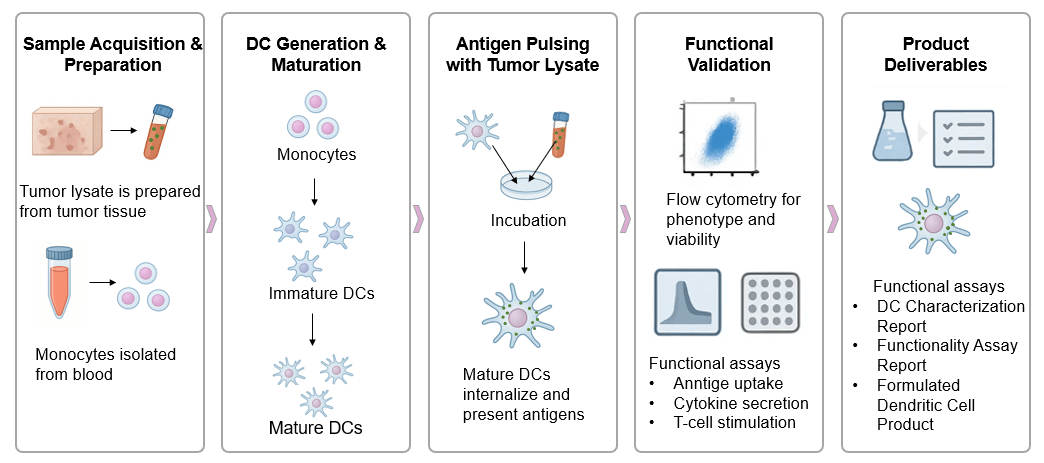
Our service offers a sophisticated platform for generating highly potent DCs loaded with a vast repertoire of tumor antigens. The core innovation lies in our strategy of pulsing DCs with whole tumor lysates, rather than relying on synthetic peptides or recombinant proteins. Lysates capture the full spectrum of tumor-associated and tumor-specific antigens, including those that are weakly immunogenic, novel, or unknown, thereby providing a more comprehensive antigenic fingerprint of the tumor.
Optimized Tumor Lysate Preparation
Targeted DC Maturation
Comprehensive Antigen Characterization
Rigorous Immunomonitoring & Functional Assays
Q1: What are the primary advantages of using whole tumor lysate for DC pulsing compared to defined peptide antigens?
A1: Whole tumor lysate offers a comprehensive and broad-spectrum antigen presentation, and subdominant epitopes that might otherwise be missed. This reduces the risk of immune escape and can elicit a more robust, poly-clonal immune response. It also circumvents the complex and costly process of individual neoantigen identification.
Q2: Is Creative Biolabs' broad-spectrum tumor antigen DC development service compatible with standard chemotherapy regimens?
A2: Yes, preclinical and some clinical evidence suggest excellent compatibility. Studies, including our own internal data, indicate that tumor lysate-pulsed DC functionality is maintained even when combined with common chemotherapeutic agents. Moreover, certain chemotherapies can synergistically enhance the anti-tumor immune response.
Q3: What type of tumor samples are required for the autologous lysate pulsing service?
A3: For our autologous service, we require patient-derived tumor tissue, ideally fresh or cryopreserved surgical resections or biopsies. The quantity and quality of the tissue are crucial for optimal lysate preparation and antigen yield.
Creative Biolabs' broad-spectrum tumor antigen DC development service: Pulsing with autologous & allogeneic lysate provides a powerful and scientifically validated approach to cancer immunotherapy. By harnessing the full antigenic landscape of tumors and ensuring compatibility with existing treatments, we empower our clients to develop more effective and durable anti-tumor immune responses.
DCs are generated ex vivo, then "pulsed" with tumor-associated peptides, proteins, lysates, or neoantigens to load them with relevant antigens. After maturation, such DCs are re-infused to present antigen via MHC molecules and stimulate robust, antigen-specific T-cell responses, underpinning many cancers vaccine strategies
Genetically Engineered DC Development
DCs are genetically modified to express specific immunomodulatory proteins to enhance T-cell infiltration, activation, and efficacy in tumor environments. This approach has been shown to boost immune checkpoint blockade and elicit durable anti-tumor immune responses in resistant cancers
DCs are transfected ex vivo with in vitro-transcribed mRNA encoding tumor antigens (or immunologic adjuvants), enabling them to internally synthesize, process, and present these antigens via MHC pathways. This method efficiently activates antigen-specific CD4+ and CD8+ T cells and has become a promising platform for cancer immunotherapy
Ready to advance your cancer immunotherapy project? Our expert team is eager to discuss your specific needs and how our broad-spectrum tumor antigen DC development service can accelerate your research or clinical goals.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION