The vaccine has made great strides in preventing disease, but it still contains microbial infections. Therefore, it is very urgent to establish a standard microbial testing platform for the vaccine. Creative Biolabs is a world leader in vaccine analysis and development. With our rich experience and advanced platform, we also provide cancer vaccine purity testing, cancer vaccine identity testing, and cancer vaccine quantity and stability testing, we are confident to provide the best development services for vaccine microbiological testing. We guarantee the best results for our customers all over the world.
By combining the traditional microbiological detection principle and different detection methods, we use advanced microbiological detection instruments and equipment, which are gradually widely used in the field of medical microbiological detection and scientific research. Our anti-interference microorganism culture medium, new biochemical identification tube, microorganism counting card, and environmental quality detection kit can facilitate multiple detections. The monitoring results of the total number of bacteria and the bacteria detection system are efficient and accurate.
We test the vaccine by cultivating bacteria, mycoplasma, and other microorganism pollution into tissue culture and using electron microscope techniques to detect virus pollution. Pure experimental tests such as the tests for bacteria living bacterium quantity of live attenuated vaccine itself, the total amount of bacteria, and other miscellaneous bacterium, poison to CHO cells and HeLa toxicity test of bacterial toxins vitality, to tissue culture method and cell infection, virus activity of plaque formation unit test. We also apply PCR to detect the presence of contaminating adventitious agents in the process and in the final formulation.






There are many instruments for detecting microbial populations in vaccines, the life science instruments we provide include the following:
Shaking table, microbial incubator, microscope, electron microscope, cell culture chamber, flow cytometry, enzyme labeling apparatus, biochip, high throughput sequencing instrument, all these life science instruments can be used to detect microbes in vaccines.
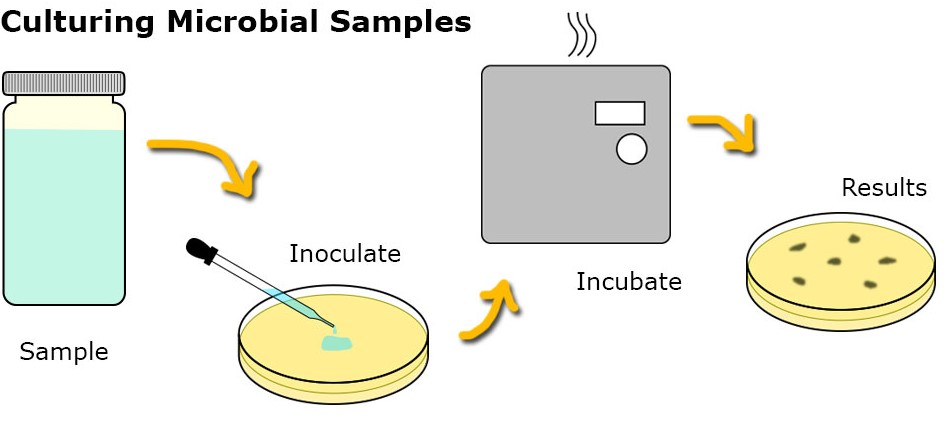 Fig.1 The Culturing Process of Microbial Tests (Creative Biolabs).
Fig.1 The Culturing Process of Microbial Tests (Creative Biolabs).
By combing relevant technologies from various disciplines, the traditional vaccine microbiological detection technology has been improved and stimulated the development of innovative technology. Creative Biolabs microbiological testing platform has applied several new technologies and also taken the advantages of the original technology. The presence and quantity of microorganisms can be monitored more efficiently than the sole traditional detection methods.
Please feel free to contact us and get more detailed information.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION