Chimeric antigen receptor (CAR) T cell therapy has emerged as a groundbreaking approach in the field of cancer immunotherapy. Despite the remarkable achievements, traditional CAR T cell therapies involve complex and costly ex vivo processes, making them inaccessible to a wide range of patients. Addressing these limitations, Creative Biolabs introduces CellRapeutics™ Viral In Vivo CAR Technology, a revolutionary platform designed to simplify and expand the reach of CAR T therapy.
Creative Biolabs' CellRapeutics™ Viral In Vivo CAR Technology focuses on generating CAR T cells directly in vivo, simplifying the in vitro manipulation process. This innovative approach utilizes advanced viral vector systems such as lentivirus and adeno-associated virus (AAV) to deliver CAR genes in vivo. This eliminates the need for complex ex vivo cell manipulations, making CAR T cell therapies more accessible and practical for clinical application. Regarding the topic of Viral In Vivo CAR, the relevant services we provide currently include customized design and synthesis of LV and AAV for in vivo CAR engineering, virus packaging, and in vivo pharmacodynamics studies, etc.
Creative Biolabs leverages both lentiviral vectors (LVs) and adeno-associated vectors (AAVs) for in vivo CAR T cell generation. Lentiviral vectors are primarily pseudotyped with the glycoprotein G of vesicular stomatitis virus (VSV-G), enabling broad tropism and high transduction efficiencies across various cell types, including activated T lymphocytes. Adeno-associated vectors, on the other hand, offer a unique platform with episomal DNA genomes, favorable for transient gene expression, particularly in proliferating cells like activated lymphocytes.
The key to Our CellRapeutics™ Viral In Vivo CAR Technology is the targeted delivery of CAR genes to T cells, which is mainly achieved through the engineering of viral vectors that modify envelope proteins to enhance T cell taxis. For example, the use of scaffold proteins and scFv ligands enhances specificity by binding to T cell surface markers such as CD3, CD4, or CD8, ensuring accurate and effective transduction.
This platform's design minimizes off-target effects and toxicity. Lentiviral vectors target T cells with high selectivity, reducing the risk of transducing non-target cells. Moreover, the improvement in vector engineering, such as the development of self-inactivating (SIN) lentiviral vectors, ensures safer integration and expression of transgenes, mitigating the risk of insertional mutagenesis and oncogenesis.
Studies have demonstrated the potential of AAV-based in vivo CART to achieve significant therapeutic outcomes in preclinical models, making it possible for more clinics to offer these treatments without needing highly specialized cell manipulation facilities.
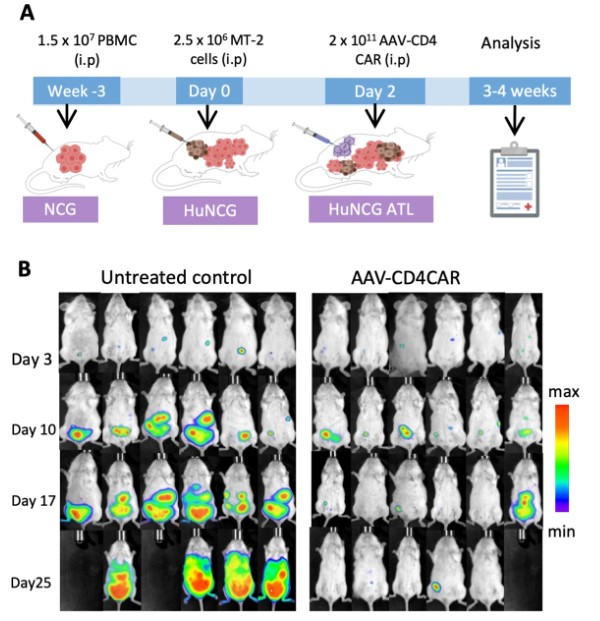 Fig.1 Cytotoxicity of AAV-based in vivo CART against tumor in mouse model.1
Fig.1 Cytotoxicity of AAV-based in vivo CART against tumor in mouse model.1
Q1: Is CellRapeutics™ Viral In Vivo CAR Technology safe?
A1: Yes, CellRapeutics™ Viral In Vivo CAR Technology employs advanced vector engineering to minimize risks, using targeted delivery mechanisms and self-inactivating vectors to ensure patient safety and therapeutic efficacy.
Q2: How does CellRapeutics™ Viral In Vivo CAR Technology improve CAR T therapy?
A2: By eliminating the need for ex vivo cell manipulation, CellRapeutics™ simplifies the CAR T cell production process, reducing costs and making therapy more accessible to a broader range of patients.
Creative Biolabs' CellRapeutics™ Viral In Vivo CAR Technology combines innovative science with practical application to deliver more effective and accessible solutions for cancer therapy.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION