Creative Biolabs delivers a glutaminolysis modulation based CD8+ T cell activation service to address critical challenges associated with T cell efficacy, suppression within the tumor microenvironment, and the optimization of adoptive cell therapies. By leveraging advanced metabolic engineering and high-throughput screening platforms, our service facilitates the robust activation of anti-tumor immunity and streamlines the drug development pipeline.
Like glycolysis, glutamine metabolism significantly increases in activated T cells and is crucial for CD8+ T cell differentiation. Interestingly, restricting glutamine metabolism during the initial activation phase of CD8+ T cells promotes the upregulation of pro-survival and memory-associated transcription factors, leading to a central effector memory phenotype when generated ex vivo. These metabolically "fit" CD8+ T cells, upon adoptive transfer, exhibit enhanced oxidative phosphorylation (OXPHOS) and spare respiratory capacity (SRC), proving highly effective in eliminating solid tumors in animal models. This highlights that strategically modulating glutaminolysis can be a potent approach to enhance CD8+ T cell activation and foster the development of highly effective, long-lasting anti-tumor immunity.
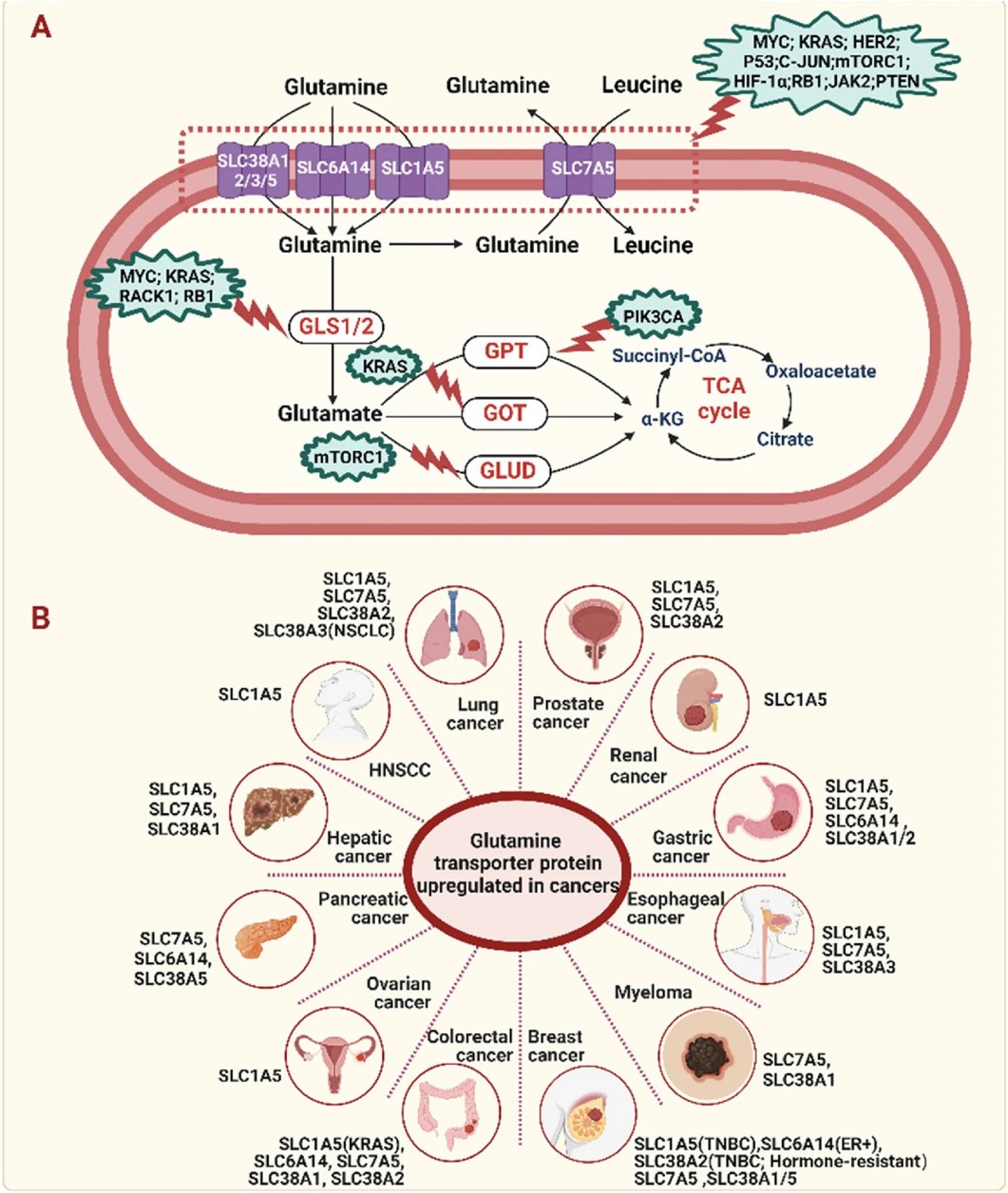 Fig.1 Cancer cells' glutamine addiction.1
Fig.1 Cancer cells' glutamine addiction.1
Creative Biolabs' glutaminolysis modulation based CD8+ T cell activation service provides specialized solutions to enhance the anti-tumor function of CD8+ T cells, leading to more effective immunotherapies. We offer a tailored approach to identify and validate novel targets, screen for effective modulators, and optimize T cell activation protocols. Our service is designed to deliver actionable insights and tangible results that directly advance your research and development pipeline.
Step 1. Metabolic Profiling and Target Identification
We conduct an initial assessment of glutamine metabolism in provided T cell samples under various conditions, utilizing metabolic flux analysis, enzyme activity assays, and gene expression profiling to identify dysregulated glutaminolysis enzymes or transporters as promising targets for modulation.
Step 2. Modulator Screening and Validation
We perform high-throughput screening of compound libraries or genetic constructs to identify and validate effective modulators of glutaminolysis, assessing their efficacy through dose-response curves and specificity assays.
Step 3. CD8+ T Cell Activation and Functional Assays (in vitro)
We treat CD8+ T cells with identified modulators and rigorously assess their activation markers, proliferation, cytokine production, and cytotoxic activity against target tumor cells in vitro to demonstrate enhanced T cell function.
Step 4. Mechanism of Action Elucidation
We conduct detailed investigations into the molecular mechanisms by which glutaminolysis modulation impacts T cell metabolism and function, employing transcriptomics, proteomics, metabolomics, and signaling pathway analyses to comprehensively understand the metabolic reprogramming and improved anti-tumor responses.
Step 5. In Vivo Efficacy Assessment (Optional)
We test the most promising modulation strategies in relevant In Vivo tumor models, involving the adoptive transfer of modulated T cells or systemic compound administration, followed by tumor growth monitoring, survival analysis, and immune cell infiltration assessment to validate therapeutic potential.
Q1: What materials do I need to provide to start a project with Creative Biolabs?
A1: To initiate your project, we typically require immune cell samples (e.g., purified CD8+ T cells or patient-derived TILs), any specific glutaminolysis inhibitors, activators, or gene targets you wish to test, and any preliminary data related to T cell activation or metabolic profiles. This helps us tailor the service precisely to your research needs. If you have any questions about material preparation, please contact our team for further discussion.
Q2: What kind of data can I expect from this service, and how will it help my project?
A2: You will receive comprehensive data reports including metabolic flux analysis, T cell activation marker expression, proliferation assays, cytokine profiles, and cytotoxic activity measurements. This data will provide clear evidence of enhanced T cell function and mechanistic insights, enabling you to make informed decisions and accelerate your therapeutic development.
Q3: How does Creative Biolabs ensure the specificity and safety of glutaminolysis modulation?
A3: We employ rigorous validation steps, including off-target screening and toxicity assessments, to ensure that any identified modulators or strategies are highly specific to the glutaminolysis pathway and maintain T cell viability. Our expertise in cell biology and pharmacology allows us to design experiments that minimize potential adverse effects while maximizing therapeutic impact.
Creative Biolabs offers a comprehensive suite of services to accelerate your cell therapy development. Beyond glutaminolysis modulation, we specialize in targeting various metabolic pathways to optimize CD8+ T cell function for enhanced immunotherapy outcomes. Our services include:
Boost your cancer immunotherapy research with Creative Biolabs' glutaminolysis modulation-based CD8+ T cell activation service. We empower you to enhance T cell function and overcome tumor-induced suppression, accelerating the development of groundbreaking cancer therapies. Get in touchtoday to discuss your project!
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION