Chimeric Antigen Receptor (CAR) T-cell therapy represents a breakthrough approach in oncology with significant efficacy in hematological malignancies. However, solid tumors pose distinctive challenges due to their heterogeneous nature, immunosuppressive microenvironment, and the prevalence of intracellular tumor-associated antigens (TAAs). Traditional CAR therapies rely on surface-expressed TAAs and are inadequate in addressing these intracellular targets. To overcome these limitations, Creative Biolabs has developed CellRapeutics™ Hybrid CAR, an innovative solution that leverages both the specificity of traditional CAR-T cells and the versatility of T-cell receptor (TCR) recognition capabilities to target intracellular TAAs.
At Creative Biolabs, we provide comprehensive Hybrid CAR development services meticulously tailored to meet the specific demands of your research objectives. Our proprietary Hybrid-TCR technology integrates the constant regions of TCR beta and alpha chains with the variable heavy (VH) and light (VL) domains of a single-chain variable fragment (scFv) antibody. This design capitalizes on the adeptness of TCRs in recognizing peptide-major histocompatibility complex (pMHC) complexes, thereby allowing the targeting of intracellular antigens such as MAGE-A4.
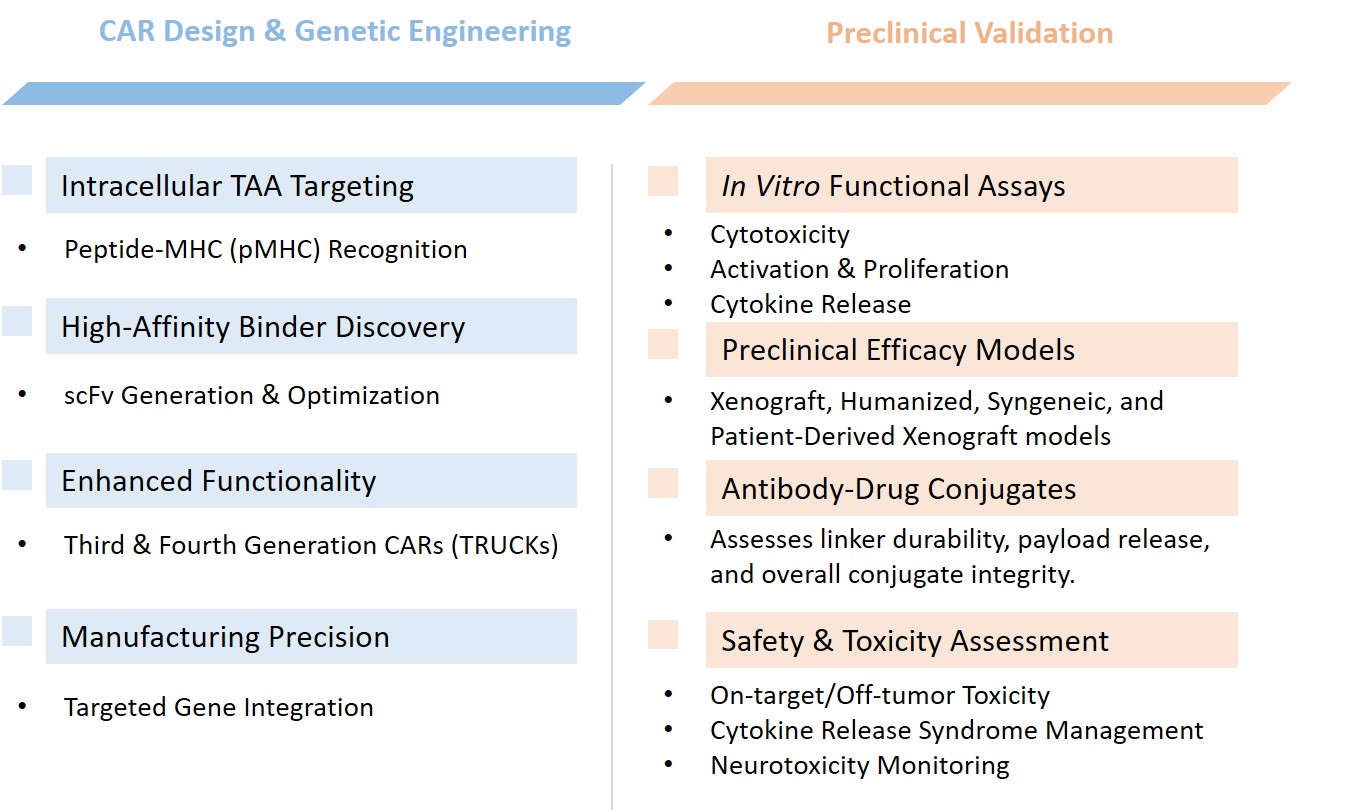
Our Hybrid CAR development services include the following specific processes:
Antigen Identification and SelectionThe initial steps involve the identification of intracellular TAAs that are implicated in solid tumors. Our scientific team ensures the tumor specificity and immunogenicity of these antigens through rigorous validation.
Utilizing multiple screening libraries we already have, such as advanced phage display libraries, to generate high-affinity scFv that can accurately identify peptide /HLA complexes. We will further optimize these scFvs to ensure stability and binding efficacy.
The optimized scFv is then fused with TCR constant regions. This entails strategic molecular cloning techniques to ensure optimal assembly and expression.
Engineered Hybrid CAR T cells undergo rigorous functional assays including cytotoxicity tests against antigen-positive and negative targets, cytokine release assays, and proliferation assessments, and several in vivo efficacy studies.
Creative Biolabs' Hybrid CAR exhibits several distinctive features that make it superior to conventional CAR designs, particularly for solid tumor applications.

Creative Biolabs' CellRapeutics™ Hybrid CAR engineering strategies offer a promising avenue for targeting the elusive challenge of solid tumors. Please feel free to contact our technical team to learn more information.
The CellRapeutics™ hybrid CAR engineering strategy is an integrated, end-to-end platform designed to overcome the core challenges of solid tumors- namely, the inaccessible nature of intracellular targets and the hostile tumor microenvironment (TME).
How does a hybrid CAR overcome the limitations of a standard TCR-T cell?
Traditional TCR-T cells depend on native signaling, which can be weaker and prone to TCR mispairing. The hybrid CAR uses a pMHC-targeting scFv linked to potent CAR signaling domains (CD3ζ and co-stimulatory motifs), ensuring stronger, more specific activation and enhanced persistence.
Can your Hybrid CAR platform be applied to "off-the-shelf" allogeneic therapies?
Absolutely. Our use of gene editing technology for targeted integration is inherently aligned with allogeneic development, as it allows us to simultaneously knock out the endogenous TCR to prevent graft-versus-host disease (GvHD) while knocking in the Hybrid CAR. We offer a dedicated service for developing allogeneic Hybrid CAR candidates.
Why use targeted integration instead of viral transduction?
Targeted integration inserts the CAR precisely into safe, high-expression loci, producing uniform expression and stable function while avoiding random insertion risks and improving batch consistency.
Blastoma-Specific CAR Construction Service develops CARs precisely targeting blastoma-associated antigens, enabling selective tumor recognition and potent cytotoxicity while minimizing off-target effects.
Carcinoma-Specific CAR Construction Service engineers CARs tailored to carcinoma markers, enhancing immune precision, infiltration, and antitumor efficacy across diverse epithelial malignancies.
Sarcoma-Specific CAR Construction Service designs CARs for sarcoma-specific antigens, delivering robust, targeted immune responses against mesenchymal tumors with improved safety and therapeutic control.
Creative Biolabs' CellRapeutics™ hybrid CAR engineering strategy provides the precision, potency, and safety controls required to finally conquer solid tumors. By targeting the vast, previously untapped repertoire of intracellular antigens and optimizing T-cell function within the hostile TME, we are ready to accelerate your most challenging immunotherapy projects. For detailed information on the CellRapeutics™ hybrid CAR engineering strategy, including customized pricing, technical discussions, and regulatory support, please contact our expert team today.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION