Struggling with tough, solid tumors, worrying about side effects, or finding your CAR-T cells just aren't sticking around? Don't sweat it! Our blastoma-specific CAR construction service helps you expedite the process and deliver safer, more effective treatments. We utilize advanced CAR engineering and rigorous testing, providing comprehensive, customized solutions to address blastoma challenges from start to finish.
Blastoma is a cancer arising from embryonic tissue, more common in children than adults. Based on tumor location, subtypes include medulloblastoma, hepatoblastoma, nephroblastoma, pleuropulmonary blastoma, retinoblastoma, neuroblastoma, and osteoblastoma. Symptoms vary, and treatment often involves surgery, radiation, and chemotherapy, which may cause long-term side effects. Adoptive cell therapy targeting blastoma-specific antigens offers a promising alternative. The chimeric antigen receptor (CAR)-modified T or NK cells have already entered clinical trials for blastoma treatment.
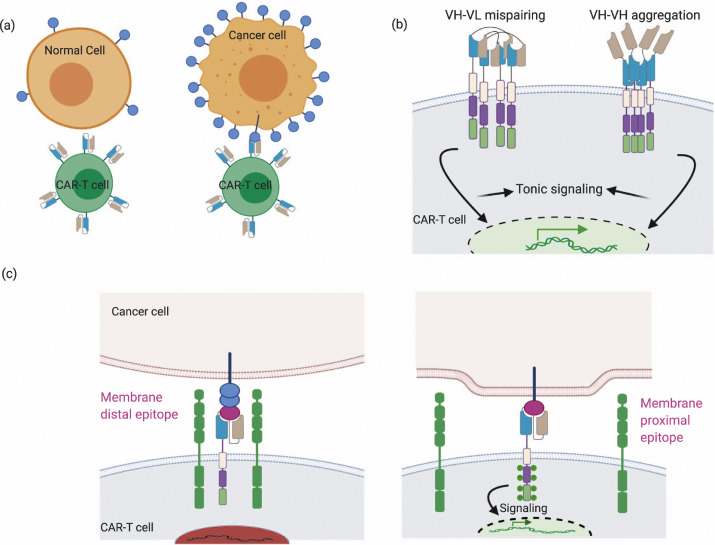 Fig.1 Design of a single-chain variable fragment in CAR-T cells.1
Fig.1 Design of a single-chain variable fragment in CAR-T cells.1
Creative Biolabs' blastoma-specific CAR construction service delivers bespoke CAR constructs and expertly engineered T cells, providing you with critical solutions to advance your oncology projects. We address key challenges in solid tumor targeting, offering meticulously designed CARs with enhanced specificity and functionality. Our service provides comprehensive preclinical data, empowering you to make informed decisions and accelerate your therapeutic candidates toward clinical translation.
Creative Biolabs has many years of experience in CARs construction for targeting blastoma-specific antigens. We can provide highly customized and best-in-class CAR products according to our special and strict requirements.
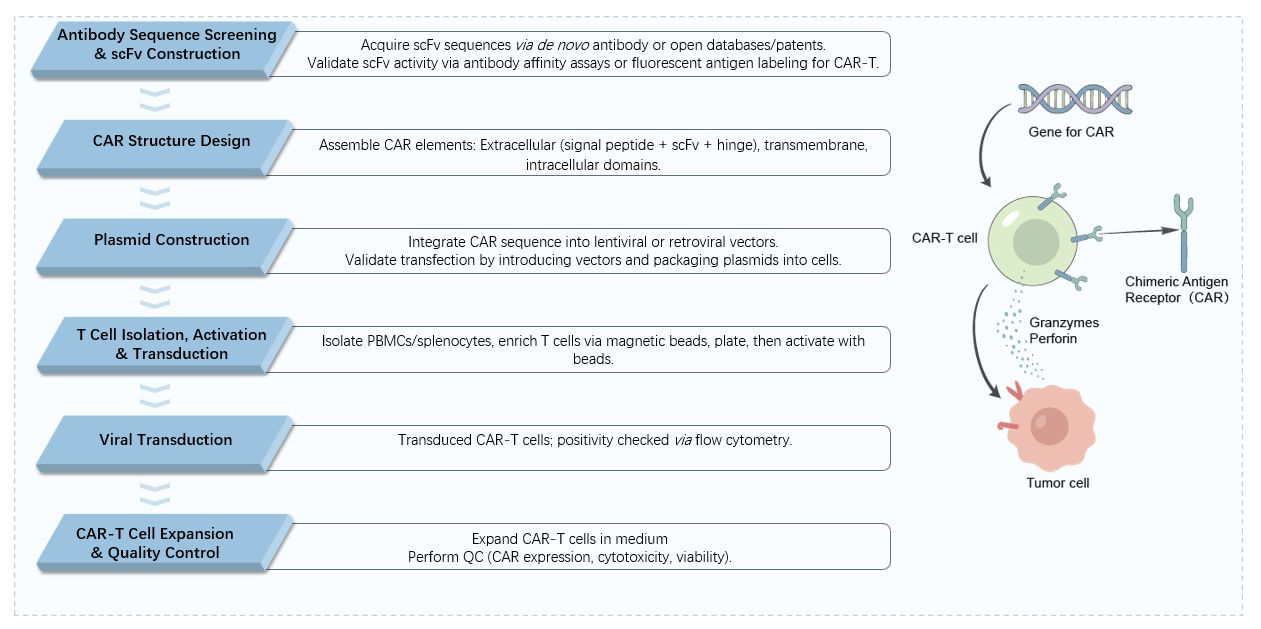
Q1: What types of blastoma antigens can your service target?
A: We can target a wide range of blastoma-specific antigens, including established markers and novel neo-antigens identified through advanced genomic analysis. Our team performs thorough validation to ensure optimal specificity and minimal off-target expression, crucial for solid tumor applications.
Q2: How do you ensure the safety of the constructed CARs for solid tumor applications?
A: Safety is paramount in our CAR design. We incorporate advanced safety features such as conditional suicide genes for rapid elimination of CAR-T cells in case of severe toxicity, and regulatable CAR expression systems for dose-dependent control. For enhanced precision, we also offer dual receptor CAR designs that require two distinct antigen recognitions for full activation, significantly reducing the risk of off-target effects.
Q3: Can you work with patient-derived T cells or only cell lines for CAR engineering?
A3: Our service is highly flexible and can accommodate various starting materials. We routinely work with both patient-derived peripheral blood mononuclear cells (PBMCs) or splenocytes for preclinical models, as well as established T cell lines, depending on your specific project requirements and the stage of your research.
Creative Biolabs is dedicated to accelerating the development of life-changing immunotherapies. Our blastoma-specific CAR construction service stands as a testament to our commitment to precision, quality, and innovation in the fight against aggressive solid tumors. By leveraging our deep expertise and state-of-the-art platforms, we empower our partners to achieve breakthroughs faster and more efficiently.
"Using Creative Biolabs' blastoma-specific CAR construction service in our glioblastoma research has significantly improved the specificity of our CAR constructs, reducing concerns about off-target effects compared to previous general approaches. Their dual receptor system design was particularly impactful." – 2024, An Sh
"The end-to-end workflow provided by Creative Biolabs, from scFv development to comprehensive QC, greatly facilitated our neuroblastoma CAR-T project. The detailed protocols and consistent results saved us considerable time and resources." – 2023, La Mr
Ready to advance your blastoma therapy project? Our expert team is here to provide detailed information and discuss how our tailored solutions can meet your specific needs.
For more information or to discuss your specific project, please reach out to our team.
Please note: If you do not find the wanted antigen, please feel free to contact us. Our highly skilled staff is very glad to help you.
| Associated malignancy | Target antigen | Receptor type | Product |
|---|---|---|---|
| Glioblastoma | IL13RA2 | scFv-CD28-OX40-CD3ζ | CAR-T-3-M401-2XZ |
| EGFRvIII | scFv-CD28-41BB-CD3ζ | CAR-LC056 | |
| IL13Rα2 | scFv-CD28-41BB-CD3ζ | CAR-LC236 | |
| CSPG4 | scFv-CD28-CD3ζ | CAR-MZ116 | |
| IL13Rα2 | IL13 mutein E13K-CD28-CD3ζ | CAR-MZ238 | |
| Hepatoblastoma | GPC3 | scFv-CD28-OX40-CD3ζ | CAR-T-3-L342-2XZ |
| HBV | scFv-CD28-41BB-CD3ζ | CAR-T-3-M319-2BZ | |
| ErbB2 | VHH-CD28-OX40-CD3ζ | CAR-T-3-M550-2XZ | |
| Glypican-3 (GPC3) | scFv-CD28-41BB-CD3ζ | CAR-LC180 | |
| Neuroblastoma | GD2 | scFv-CD28-41BB-CD3ζ | CAR-NK-3-M330-2BZ |
| CD171 | scFv-CD28-41BB-CD3ζ | CAR-T-3-M307-2BZ |
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION