All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Here are the HLA-DR CAR products we offer you, including HLA-DR CAR vector products, CAR cell products, CAR mRNA.
HLA-DR is a major histocompatibility complex (MHC) molecule of human class II containing two subunits (a and β). It is constitutively expressed on the surface of monocytes, macrophages, and B lymphocytes and presents antigens to CD4+ T cells. Most T cells do not express HLA-DR, and only a subset of activated T cells express HLA-DR during the later stages of the T cell response to the immune response. HLA-DR is co-expressed with CD1a in epidermal Langerhans cells.
HLA-DR is used for the enumeration of B lymphocytes and monocytes in peripheral blood; identification of lymphoma and leukemia; Expression of HLA-DR antigen on activated T lymphocytes and dendritic cell subsets in peripheral blood and lymphoid tissues.
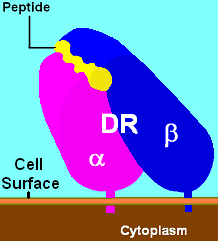 Fig.1 Schematic diagram of DR and binding ligand (yellow). (Wikipedia)
Fig.1 Schematic diagram of DR and binding ligand (yellow). (Wikipedia)
Acute B-lymphoblastic Leukemia
T-lymphocytic leukemia
Creative Biolabs focuses on the research and product development of tumor immunotherapy, providing design, customization and development services for HLA-DR CAR-T cells. Figure 1 shows a second-generation CAR construct designed using an anti-HLA-DR antibody.
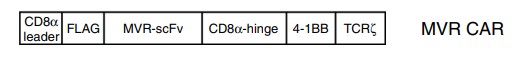 Fig.2 Schematic diagram of the design of the HLA-DR (MVR) CAR.1
Fig.2 Schematic diagram of the design of the HLA-DR (MVR) CAR.1
Anti-HLA-DR CAR-T Expression Test
Based on years of technical experience, Creative Biolabs has developed unique fluorescently site-directed tagged protein products to facilitate the development of cell therapies. Figure 2 shows the flow cytometry results of CAR expression levels on the surface of HLA-DR CAR T cells.
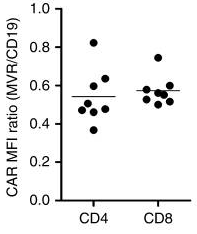 Fig.3 Flow cytometry was used to analyze the expression of HLA-DR.1
Fig.3 Flow cytometry was used to analyze the expression of HLA-DR.1
Anti-HLA-DR CAR-T Viability Study
The limited replicational lifespan of CAR-T cells is a major obstacle associated with current CAR-T cell therapy. Therefore, the viability and bio-distribution studies of CAR-T are of great significance for the development of CAR-T therapies. As a leading provider in the field of CAR-T development, Creative Biolabs now provides customers with testing services including in vivo proliferation, survival, migration and distribution of T cells, and T cell persistence.
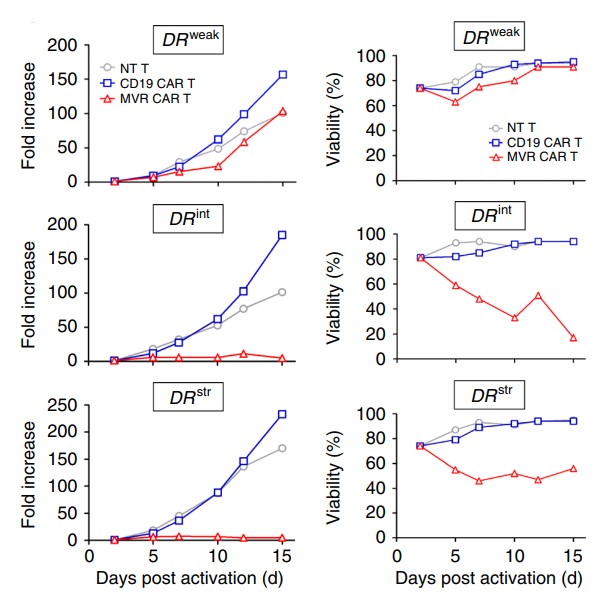 Fig.4 Growth and viability of HLA-DR CAR.1
Fig.4 Growth and viability of HLA-DR CAR.1
Anti-HLA-DR CAR-T In Vitro Cytotoxicity Assay
In addition to providing cytokine release to measure CAR-T cell activity, Creative Biolabs also assesses the killing activity of CAR-T cells by assays of specific cell lysis in targeted tumor cell populations.
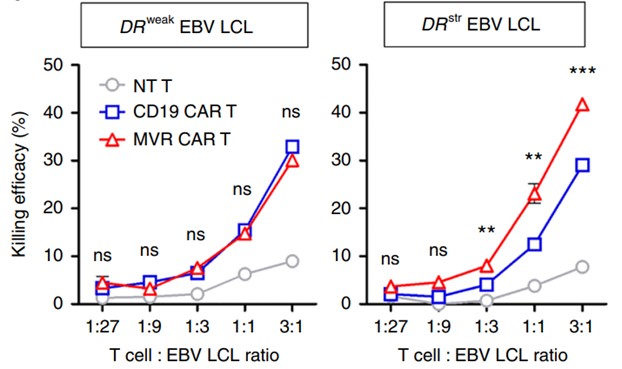 Fig.5 Cytotoxicity assay of HLA-DR CAR T cells.1
Fig.5 Cytotoxicity assay of HLA-DR CAR T cells.1
Anti-HLA-DR CAR-T Cell Therapy Animal Models
Creative Biolabs offers xenograft models to evaluate in vivo HLA-DR CAR T cell efficacy. The figure below shows the flowchart of an in vivo study of HLA-DR CAR T cells in a xenograft mouse model.
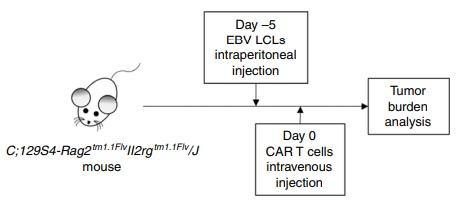 Fig.6 HLA-DR CAR T cells in vivo evaluation procedure.1
Fig.6 HLA-DR CAR T cells in vivo evaluation procedure.1
Efficacy Test of Anti-HLA-DR CAR-T
Creative Biolabs is committed to the development of tumor immunotherapy technology and has established a complete set of CAR-T in vivo pharmacodynamics experimental platforms to provide technical support and professional CAR-T services for CAR-T in vivo experiments. Figure 7 shows in vivo imaging of HLA-DR CAR T cells in a mouse model.
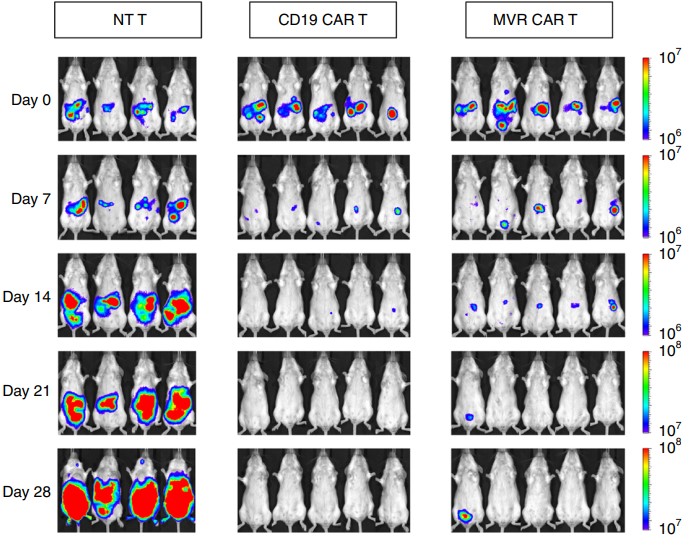 Fig.7 Tumor burden detection in transplanted mice.1
Fig.7 Tumor burden detection in transplanted mice.1
Anti-HLA-DR CAR-T Cytokine Release Test
CAR-T cell quality testing mainly includes biochemical testing, microbial testing and cytology testing. Cytokine ELISA assays and flow cytometry are important ways to measure their activity and components.
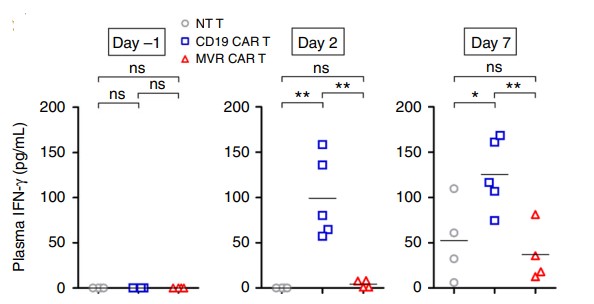 Fig.8 IFN-γ levels following in vivo infusion of HLA-DR CAR T cells.1
Fig.8 IFN-γ levels following in vivo infusion of HLA-DR CAR T cells.1
Reference
 Loading...
Loading...
| CAT | Product Name | Target Species | Antibody Clone | Antibody Host | Receptor Construction | Vector Type | Targeting Cell Type | CAR Vector Type | Inquiry & Datasheet |
| CAR-ZP081 | Anti-HLA-DR (MVR) h(41BB-CD3ζ) CAR, pCDCAR1 | Human | MVR | Mouse | scFv-4-1BB-CD3ζ | Lentiviral vector | T cell | ||
| CAR-ZP082 | Anti-HLA-DR (MVR) h(CD28-CD3ζ) CAR, pCDCAR1 | Human | MVR | Mouse | scFv-CD28-CD3ζ | Lentiviral vector | T cell | ||
| CAR-0120ZP219 | Anti-HLA-DR (huLym-1-B) h(CD28-CD3ζ) CAR, pCDCAR1 | Human | huLym-1-B | Humanized | scFv-CD28-CD112ζ | Lentiviral vector | T Cell | ||
| CAR-0120ZP220 | Anti-HLA-DR (huLym-1-B) h(4-1BB-CD3ζ) CAR, pCDCAR1 | Human | huLym-1-B | Humanized | scFv-4-1BB-CD112ζ | Lentiviral vector | T Cell | ||
| CAR-0120ZP221 | Anti-HLA-DR (CB58YJ) h(CD28-CD3ζ) CAR, pCDCAR1 | Human | CB58YJ | Mouse | scFv-CD28-CD113ζ | Lentiviral vector | T Cell | ||
| CAR-0120ZP223 | Anti-HLA-DR (DR4B117) h(4-1BB-CD3ζ) CAR, pCDCAR1 | Human | DR4B117 | Human | scFv-CD28-CD114ζ | Lentiviral vector | T Cell | ||
| CAR-0120ZP224 | Anti-HLA-DR (DR4B117) h(CD28-CD3ζ) CAR, pCDCAR1 | Human | DR4B117 | Human | scFv-4-1BB-CD114ζ | Lentiviral vector | T Cell | ||
| CAR-0120ZP225 | Anti-HLA-DR (L243) h(4-1BB-CD3ζ) CAR, pCDCAR1 | Human | L243 | Mouse | scFv-CD28-CD115ζ | Lentiviral vector | T Cell | ||
| CAR-0120ZP226 | Anti-HLA-DR (L243) h(CD28-CD3ζ) CAR, pCDCAR1 | Human | L243 | Mouse | scFv-4-1BB-CD115ζ | Lentiviral vector | T Cell | ||
| CAR-0120ZP227 | Anti-HLA-DR (1D09C3) h(4-1BB-CD3ζ) CAR, pCDCAR1 | Human | 1D09C3 | Human | scFv-CD28-CD116ζ | Lentiviral vector | T Cell | ||
| CAR-0120ZP228 | Anti-HLA-DR (1D09C3) h(CD28-CD3ζ) CAR, pCDCAR1 | Human | 1D09C3 | Human | scFv-4-1BB-CD116ζ | Lentiviral vector | T Cell | ||
| XS-0622-ZP3322 | Anti-HLA-DR h(VHH1-VHH2-CD28-CD3ζ) Biepitopic CAR, pCDCAR1 | Human | VHH1-VHH2-CD28-CD3ζ | Lentiviral vector | T Cell | ||||
| XS-0622-ZP3494 | Anti-HLA-DR h(VHH1-VHH2-4-1BB-CD3ζ) Biepitopic CAR, pCDCAR1 | VHH1-VHH2-4-1BB-CD3ζ | Lentiviral vector | T Cell | |||||
| XS-0822-YF514 | Anti-Human HLA-DR (XW-514) h(41BB-CD3ζ) CAR IVT Plasmid, pCARIVT | Human | XW-514 | Mouse | scFv-41BB-CD3ζ | In Vitro Transcription (IVT) Vector | |||
| XS-0822-YF1434 | Anti-Human HLA-DR (XW-514) h(CD28-CD3ζ) CAR IVT Plasmid, pCARIVT | Human | XW-514 | Mouse | scFv-CD28-CD3ζ | In Vitro Transcription (IVT) Vector | |||
| XS-0822-YF2354 | Anti-Human HLA-DR (XW-514) h(CD28-41BB-CD3ζ) CAR IVT Plasmid, pCARIVT | Human | XW-514 | Mouse | scFv-CD28-41BB-CD3ζ | In Vitro Transcription (IVT) Vector | |||
| XS-1122-YF4194 | Anti-HLA-DR TCR-ABR (scFv-CD3δ, XW-514) CAR Plasmid, pCDCAR1 | Human | XW-514 | Mouse | scFv-CD3δ | Lentiviral vector | T Cell | ||
| XS-1122-YF6034 | Anti-HLA-DR (XW-514) h(CD28-CD3ζ) CAR, pAAV | Human | XW-514 | Mouse | scFv-CD28-CD3ζ | Adeno-associated viral (AAV) vector | T Cell | ||
| XS-1122-YF6954 | Anti-HLA-DR (XW-514) h(41BB-CD3ζ) CAR, pAAV | Human | XW-514 | Mouse | scFv-41BB-CD3ζ | Adeno-associated viral (AAV) vector | T Cell | ||
| XS-1122-YF7874 | Anti-HLA-DR (XW-514) h(CD28-41BB-CD3ζ) CAR, pAAV | Human | XW-514 | Mouse | scFv-CD28-41BB-CD3ζ | Adeno-associated viral (AAV) vector | T Cell |
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION