Beyond Prevention: The Science of Therapeutic HPV Vaccines Explained
Human papillomavirus (HPV) remains one of the most significant global health challenges, linked to a range of cancers and pre-cancerous conditions. While preventive vaccines have made strides in reducing new infections, they leave a critical gap: they offer no benefit to those already infected or living with HPV-related lesions. This is where therapeutic HPV vaccines step in—a cutting-edge field of research focused on treating existing infections and stopping disease progression. Let's explore the science, progress, and promise of these innovative treatments.
The Dual Burden of HPV and the Need for Therapeutic Solutions
HPV-related diseases pose a dual challenge to global health. On one hand, preventive vaccines have revolutionized public health by protecting against the most high-risk HPV types, but their effectiveness is limited to those who receive them before exposure. They cannot reverse existing infections, shrink pre-cancerous lesions, or treat established cancers. On the other hand, the global burden of HPV-related illness remains staggering: each year, over 600,000 new cases of cervical cancer—caused primarily by HPV—are diagnosed worldwide, highlighting an urgent need for treatments that can help those already affected.
Therapeutic HPV vaccines represent a paradigm shift. Unlike preventive vaccines, which aim to block initial infection, therapeutic vaccines work by activating the immune system to target and eliminate cells already harboring HPV. Their core mechanism focuses on two key viral proteins: E6 and E7. These proteins are produced by high-risk HPV types and play a critical role in cancer development by disrupting normal cell growth and preventing infected cells from being destroyed by the immune system. By training the immune system to recognize and attack cells producing E6 and E7, therapeutic vaccines aim to clear infections, shrink pre-cancerous lesions, and reduce the need for invasive procedures like surgical excision—ultimately lowering recurrence risks.
The Landscape of Therapeutic HPV Vaccine Technologies
Researchers are exploring multiple approaches to develop effective therapeutic HPV vaccines, each with unique strengths and challenges. Let's break down the most promising platforms.
DNA-Based Vaccines: Pioneers in Clinical Development
DNA vaccines have emerged as front-runners in therapeutic HPV research. These vaccines use small, circular pieces of DNA (plasmids) engineered to carry genes for the HPV E6 and E7 proteins. When delivered into the body, cells take up the DNA, produce the viral proteins, and present them to the immune system, triggering a targeted response.
A key advantage of DNA vaccines is their ability to induce strong, long-lasting T-cell responses—the immune cells responsible for hunting down and destroying infected or cancerous cells. To enhance their effectiveness, researchers have developed delivery techniques like electroporation, which uses mild electrical pulses to create tiny pores in cell membranes, allowing the DNA plasmids to enter cells more efficiently. Early clinical trials using this approach have shown promising results, with significant rates of lesion regression and viral clearance in participants with pre-cancerous cervical lesions.
Another innovation in DNA vaccine design involves combining E6/E7 plasmids with genes that produce immune-boosting molecules, such as certain cytokines. These molecules help strengthen the “Th1-type” immune response—a specific branch of immunity critical for fighting intracellular pathogens like viruses and cancer cells. Early studies combining these enhanced DNA vaccines with other immune therapies have reported improved response rates, with some showing up to 27.6% of participants experiencing significant lesion shrinkage.
Viral Vector and Peptide Vaccines: Alternative Approaches
Beyond DNA vaccines, viral vector vaccines are being explored as another therapeutic strategy. These vaccines use harmless viruses (like adenoviruses) that have been modified to carry the genes for HPV E6 and E7. When the vector enters cells, it delivers these genes, prompting the production of viral proteins and triggering an immune response. Animal studies have shown that viral vector vaccines can induce durable immune memory, meaning the immune system retains the ability to recognize and attack HPV-infected cells long after vaccination.
Peptide vaccines represent a third approach. These vaccines use short fragments (peptides) of the E6 or E7 proteins, rather than full genes. When combined with adjuvants—substances that enhance immune responses—peptide vaccines can stimulate T cells to target HPV-infected cells. Early-phase trials of peptide vaccines targeting HPV16 E7 have demonstrated good safety profiles, with participants showing increased T-cell activity against the virus, a key indicator of potential effectiveness.
Services you may interested in
Key Clinical Trials: Milestones in Translation to Patient Care
The journey from lab to clinic for therapeutic HPV vaccines relies on rigorous clinical trials, and recent years have seen significant progress in late-stage research.
Phase III Progress: A Potential First Approval
One leading DNA vaccine candidate has completed a large-scale Phase III trial, marking a critical milestone. This trial enrolled participants with high-grade pre-cancerous cervical lesions (CIN2/3) in a double-blind, placebo-controlled, global study. The primary goal was to evaluate a “composite endpoint”: whether the vaccine could achieve both histological regression (shrinking or disappearance of lesions) and viral clearance (elimination of HPV from the body) by week 36.
Follow-up of all participants was completed in March 2025, with top-line results expected by the end of the year. If successful, this vaccine could become the first approved therapeutic HPV vaccine, offering a non-surgical option for millions living with pre-cancerous lesions.
Combination Therapies: Enhancing Immune Responses
Another promising area of research involves combining therapeutic vaccines with immune checkpoint inhibitors—drugs that help the immune system “see” and attack cancer cells. In a mid-stage trial, a therapeutic vaccine was combined with a checkpoint inhibitor targeting the PD-1/PD-L1 pathway, a mechanism cancer cells use to hide from the immune system.
In 29 evaluable participants, the combination therapy achieved a 27.6% overall response rate, with no serious immune-related side effects. Researchers believe the vaccine works by increasing T-cell infiltration into infected tissues, making the checkpoint inhibitor more effective. This synergy could be particularly valuable for treating HPV-related cancers beyond the cervix, such as head and neck cancers, where treatment options are often limited.
Molecular Mechanisms: Innovations to Boost Vaccine Effectiveness
To maximize the power of therapeutic HPV vaccines, researchers are diving deep into the molecular mechanisms of immunity, developing strategies to enhance how vaccines activate and sustain immune responses.
Adjuvants and Delivery: Supercharging Immune Activation
Adjuvants are critical for optimizing vaccine performance, and recent advances in adjuvant design have shown dramatic results. One research team, for example, modified DNA vaccine plasmids by adding multiple copies of a DNA sequence called CpG, which stimulates immune cells called dendritic cells to trigger stronger T-cell responses. When combined with a gene encoding the cytokine IL-28B—another immune booster—this approach increased the production of IFN-γ (a key anti-viral protein) by 3.8 times and significantly enhanced the expression of granzyme B, an enzyme T cells use to kill infected cells.
Delivery technology has also improved. Electroporation devices, which enhance DNA uptake into cells, have been shown to increase viral clearance rates by 40% compared to traditional injections in clinical trials. These innovations allow therapeutic vaccines to more effectively activate the immune system against HPV.
Personalized Vaccines: Tailoring Treatment to Individuals
No two immune systems or HPV infections are identical, which is why personalized vaccines are emerging as a frontier in research. These vaccines are designed based on a patient's specific HPV subtype and their unique immune profile. For example, multi-epitope vaccines target multiple fragments of E6 and E7 proteins from the specific HPV type infecting the patient. By analyzing tumor-infiltrating lymphocytes (TILs)—immune cells present in lesions—researchers can identify which viral proteins the immune system is already targeting, then design vaccines to amplify those responses. This personalized approach aims to improve treatment success by matching the vaccine to the patient's biology.
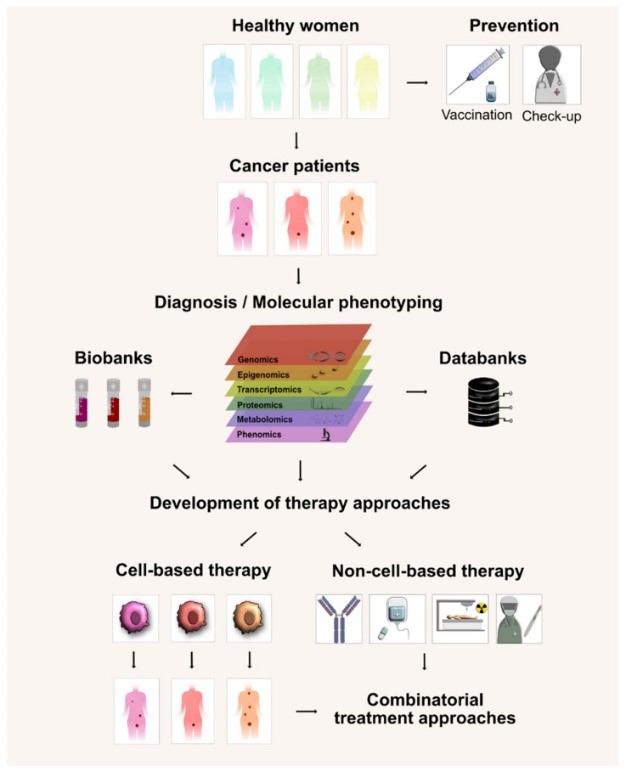 Fig.1 The Process of Personalized HPV Vaccine Therapy.1,2
Fig.1 The Process of Personalized HPV Vaccine Therapy.1,2
Challenges and Future Directions: Overcoming Hurdles
Despite progress, therapeutic HPV vaccines face significant challenges. Overcoming these will be key to realizing their full potential.
Current Bottlenecks and Solutions
One major hurdle is immune evasion: HPV has evolved strategies to hide from the immune system, such as reducing the expression of MHC-I molecules—proteins that display viral antigens to T cells. Without MHC-I, infected cells can avoid detection. To counter this, combining therapeutic vaccines with checkpoint inhibitors has shown promise, as these drugs can reverse immune evasion mechanisms and help T cells recognize infected cells.
Long-term safety is another critical consideration. DNA vaccines, in particular, require long-term follow-up to rule out rare risks like genetic toxicity. To date, late-stage trials have reported only mild side effects, such as injection site pain, suggesting a favorable safety profile, but 10+ years of data will be needed to confirm long-term safety.
Next-Generation Technologies
The future of therapeutic HPV vaccines lies in next-generation platforms. mRNA vaccines, which gained global attention during the COVID-19 pandemic, are now being adapted for HPV. Early-phase trials of mRNA vaccines targeting HPV16 and 18 have shown enhanced antigen presentation—the process by which immune cells “learn” to recognize viral proteins—suggesting they may induce stronger, faster immune responses than traditional DNA vaccines.
Multi-valent vaccines are also in development, designed to target not just HPV16 and 18 (which cause most HPV-related cancers) but also other high-risk types like 31 and 33. By covering more subtypes, these vaccines could reduce the risk of cross-infection and provide broader protection against disease progression.
A New Era in HPV Disease Management
Therapeutic HPV vaccines represent more than a medical breakthrough—they are a blueprint for precision immunotherapy. By targeting the specific proteins that drive HPV-related disease, they harness the immune system's natural ability to fight infection and cancer, offering a less invasive alternative to surgery and other treatments.
The success of these vaccines could also pave the way for similar approaches to other virus-linked cancers, such as those caused by hepatitis B virus (HBV) or Epstein-Barr virus (EBV). With late-stage trials underway and next-generation technologies in development, the 2025–2030 period could see a wave of approvals for therapeutic HPV vaccines. Combined with existing preventive strategies, these treatments may one day make HPV-related diseases a thing of the past, bringing us closer to the goal of global control by the mid-21st century.
In the end, the story of therapeutic HPV vaccines is one of scientific ingenuity and hope—proof that by understanding the intricate dance between viruses and the immune system, we can develop treatments that transform lives.
If you want to learn more about the hpv vaccine, please refer to:
The Molecular Architecture of HPV: Decoding the L1 Capsid Protein
From Virus to Vaccine: The Science Behind HPV Prevention
HPV E6/E7 Oncoproteins: Key in Therapeutic Vaccine Development
HPV Vaccine Development Timeline: From Lab Research to Clinical Application
Ready-to-Use HPV Antibodies
| CAT | Product Name | Target | Type | Price |
|---|---|---|---|---|
| VAnt-Wyb178 | HPV Monoclonal Antibody (E7, IgG2a, 3.3 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb179 | HPV Monoclonal Antibody (E7, IgG1, 3.8 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb180 | HPV Monoclonal Antibody (E7, IgG2a, 3.4 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb181 | HPV Monoclonal Antibody (E6, IgG1-κ, 4.8 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb182 | HPV Monoclonal Antibody (E6, IgG1-κ, 1.01 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb183 | HPV Monoclonal Antibody (L1, IgG1, 3.0 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb184 | HPV Monoclonal Antibody (L1, IgG2a-κ, 4.5 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb185 | HPV Monoclonal Antibody (L1, IgG2a-κ, 3.3 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb186 | HPV Monoclonal Antibody (E7, IgG2b, 4.3 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb187 | HPV Monoclonal Antibody (E7, IgG2a, 4.4 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb188 | HPV Monoclonal Antibody (E7, IgG1, 3.0 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb189 | HPV Monoclonal Antibody (E7, IgG2a, 5.1 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb190 | HPV Monoclonal Antibody (E7, IgG2a, 4.7 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb191 | HPV Monoclonal Antibody (Clone: VA-1870H, IgG1-κ, 1 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb192 | HPV Monoclonal Antibody (Clone: VA-187H, IgG1-κ, 1 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb193 | HPV Monoclonal Antibody (Clone: VA-187H, IgG1, 7.4 mg/ML) | Antibody | Inquiry |
Browse our HPV Antigen Products
→Human Papillomavirus Antigens
Need a custom solution? If our off-the-shelf products aren't a perfect fit, we can create one for you. Contact us to design a product that precisely matches your experimental demands.
References
- Polten, Robert, et al. "Towards novel gene and cell therapy approaches for cervical cancer." Cancers 15.1 (2022): 263. https://doi.org/10.3390/cancers15010263
- Distributed under Open Access license CC BY 4.0, without modification.
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


