HPV E6/E7 Oncoproteins: Key in Therapeutic Vaccine Development
The Global Challenge of HPV and Cervical Cancer
Cervical cancer remains one of the most prevalent malignancies affecting women worldwide, with a staggering epidemiological burden. Central to its pathogenesis is infection with high-risk human papillomavirus (HPV) subtypes, particularly HPV16 and HPV18, which are associated with approximately 99.7% of all cervical cancer cases. Unlike transient HPV infections that are often cleared by the immune system, persistent infections drive carcinogenesis through the sustained activity of two viral oncoproteins: E6 and E7. These proteins act as core drivers of malignant transformation, making them critical targets for therapeutic intervention.
Current preventive HPV vaccines, which target major viral subtypes, have significantly reduced the incidence of new infections and precancerous lesions. However, they possess inherent limitations: they cannot treat existing infections, nor can they eliminate HPV DNA that has integrated into the host genome—a hallmark of advanced cervical neoplasia. This unmet clinical need underscores the urgency for therapeutic vaccines. E6 and E7 oncoproteins are ideal candidates for such vaccines due to their consistent expression in tumor cells, distinguishing them from normal tissues and enabling specific immune targeting. Additionally, HPV E6/E7 mRNA detection has emerged as a valuable biomarker, aiding in cancer screening, risk stratification of precancerous lesions, and post-treatment surveillance to monitor recurrence.
Molecular Mechanisms of HPV E6/E7 Oncoproteins in Carcinogenesis
The carcinogenic potential of HPV E6 and E7 lies in their ability to disrupt key cellular regulatory pathways, promoting uncontrolled proliferation and evading apoptosis.
E6: Disabling Tumor Suppression and Promoting Immortality
E6 exerts its oncogenic effects primarily through the degradation of the tumor suppressor protein p53. By recruiting the E6-associated protein (E6AP), a ubiquitin ligase, E6 facilitates the ubiquitination and subsequent proteasomal degradation of p53. This inactivation of p53 impairs the cell's ability to detect DNA damage and initiate apoptosis, allowing genetically damaged cells to survive and proliferate. Furthermore, E6 upregulates the expression of human telomerase reverse transcriptase (hTERT), the catalytic subunit of telomerase. Telomerase activation maintains telomere length in rapidly dividing cells, conferring cellular immortality—a critical step in the progression from precancer to invasive carcinoma.
E7: Driving Cell Cycle Dysregulation
E7's oncogenic activity centers on its interaction with the retinoblastoma protein (Rb), a key regulator of the cell cycle. E7 binds to hypophosphorylated Rb, disrupting its association with the E2F family of transcription factors. This dissociation releases E2F, which then activates the transcription of genes required for cell cycle progression (e.g., cyclins), driving cells to enter the S phase prematurely and bypassing critical checkpoints. Beyond Rb inactivation, E7 also modulates epigenetic regulation by altering histone modifications, such as histone acetylation and methylation, to upregulate the expression of oncogenes and downregulate tumor suppressors, further promoting malignant transformation.
Immune Evasion: Escaping Host Surveillance
To establish persistent infections and support tumor growth, HPV has evolved mechanisms to evade the host immune system, with E6 and E7 playing pivotal roles. One key strategy is the downregulation of major histocompatibility complex class I (MHC-I) molecules on the surface of infected cells. By inhibiting MHC-I expression and antigen presentation pathways, E6 and E7 reduce the ability of cytotoxic T lymphocytes (CTLs) to recognize and eliminate virus-infected or transformed cells. Recent research has also identified a novel role for circular RNA derived from E7 (circE7) in immune evasion. CircE7 epigenetically suppresses the expression of Galectin-9, a molecule involved in T cell activation, thereby impairing T cell function and weakening anti-tumor immune responses.
Advances in HPV E6/E7-Targeted Therapeutic Vaccines
The consistent expression and oncogenic necessity of E6/E7 have made them prime targets for therapeutic vaccine development, with several approaches showing promising results in preclinical and early clinical studies.
mRNA Vaccines: Harnessing Nucleic Acid Technology
mRNA vaccines have emerged as a powerful platform for HPV therapy, offering flexibility in antigen design and rapid production. A key focus in antigen optimization involves codon engineering and untranslated region (UTR) modification, which enhance mRNA stability and translational efficiency, thereby increasing antigen expression in target cells. Additionally, fusing E6/E7 antigens with chemokines—small proteins that recruit immune cells—has shown remarkable efficacy. Preclinical studies demonstrate that such fusion vaccines enhance the recruitment of CTLs and helper T lymphocytes (HTLs) to tumor sites, leading to complete tumor regression in animal models.
Delivery systems are critical for mRNA vaccine efficacy, with lipid nanoparticles (LNPs) being widely used to protect mRNA from degradation and facilitate cellular uptake. Innovations in formulation, such as lyophilization (freeze-drying), address logistical challenges by eliminating the need for cold-chain storage, improving vaccine accessibility in resource-limited settings. These advances have paved the way for the clinical evaluation of mRNA vaccines targeting HPV-associated malignancies.
DNA Vaccines and Gene Editing Strategies
DNA vaccines, which deliver E6/E7-encoding plasmids to induce immune responses, have been enhanced through the integration of gene editing technologies. Zinc finger nucleases, for example, have been engineered to specifically target and cleave HPV16 E6/E7 genes in infected cells. In preclinical models, this approach has reversed precancerous lesions by eliminating the oncogenic drivers. Another promising direction is multi-epitope vaccine design, which uses bioinformatics to predict immunogenic CTL and HTL epitopes from E6/E7 proteins across multiple HPV subtypes, broadening vaccine coverage and reducing the risk of subtype-specific escape.
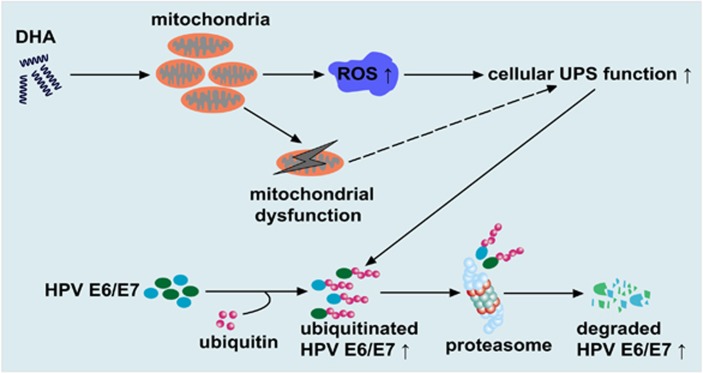 Fig.1 Model showing how DHA reduces cancer-causing E6/E7 proteins in HPV-infected cells.1, 2
Fig.1 Model showing how DHA reduces cancer-causing E6/E7 proteins in HPV-infected cells.1, 2
Services you may interested in
Combination Therapies: Enhancing Immune Responses
Combination strategies have emerged to overcome immune tolerance and improve vaccine efficacy. Immune checkpoint blockade, which reverses T cell exhaustion, has shown synergy with E6/E7 vaccines. For instance, targeting TIM-3 and PD-1—immune checkpoints upregulated in HPV-associated tumors—restores T cell activity and overcomes resistance to single-agent PD-1 inhibition. Additionally, local treatments combined with systemic vaccination have yielded encouraging results. Local interventions, such as cryotherapy to reduce tumor burden, combined with antibody-based therapies that neutralize HPV antigens, have achieved high rates of HPV clearance in clinical studies, highlighting the potential of multimodal approaches.
Technical Challenges and Future Directions
Despite significant progress, several challenges must be addressed to advance E6/E7-targeted vaccines to clinical practice.
Optimizing Immunogenicity
A major hurdle is the low immunogenicity of E6/E7 proteins, partly due to their low expression levels and intracellular localization, which limit antigen presentation to immune cells. Strategies to enhance antigen processing, such as coupling E6/E7 to heat-shock proteins or using adjuvants that activate dendritic cells (DCs), are under investigation. Novel adjuvants, including those that trigger toll-like receptors (TLRs) or modulate cytokine release, are being tested in combination with nanocarriers to improve antigen delivery and immune activation.
Improving Delivery Precision
Targeted delivery to antigen-presenting cells (APCs), such as DCs, is critical for maximizing vaccine efficacy. Approaches include fusing E6/E7 antigens with DC-specific chemokines or using monoclonal antibodies to guide vaccine particles to DCs. RNA interference (RNAi) has also been explored as an adjuvant strategy: small interfering RNAs (siRNAs) that silence E6/E7 expression can reduce oncoprotein levels, sensitizing tumors to immune-mediated destruction while enhancing vaccine-induced responses.
Accelerating Clinical Translation
Personalized vaccine design is emerging as a priority, tailoring vaccines to individual HPV subtypes and host immune profiles to improve specificity. This requires comprehensive molecular profiling of tumors to identify dominant HPV subtypes and immune biomarkers that predict response. Additionally, large-scale clinical trials are needed to evaluate combination therapies, such as vaccines paired with novel immunomodulators, in advanced HPV-associated cancers. These trials must address questions of safety, dosing, and scheduling to establish optimal treatment regimens.
HPV E6/E7 oncoproteins are indispensable targets for cervical cancer therapeutic vaccines, given their central role in carcinogenesis and consistent tumor expression. Advances in mRNA technology, gene editing, and combination therapies have advanced these vaccines toward clinical use. Integrating mRNA vaccines with gene editing may enable precise clearance of integrated HPV DNA, while AI-enhanced epitope prediction can broaden multivalent vaccine efficacy. However, challenges persist, including overcoming immune suppression in the tumor microenvironment (e.g., regulatory T cell infiltration and PD-L1 expression) and reducing production costs for global access. With interdisciplinary collaboration, E6/E7-targeted vaccines hold great potential to transform HPV-associated cancer treatment, improving patient outcomes worldwide.
Creative Biolabs specializes in developing therapeutic vaccines targeting the Human Papillomavirus (HPV) E6/E7 oncoproteins. These key proteins are the driving force behind HPV-associated cancers, such as cervical cancer. Creative Biolabs offers comprehensive services and related products, from vaccine design and development to preclinical testing, dedicated to providing innovative immunotherapy solutions for combating existing HPV infections and related malignancies.
If you want to learn more about the hpv vaccine, please refer to:
The Molecular Architecture of HPV: Decoding the L1 Capsid Protein
Beyond Prevention: The Science of Therapeutic HPV Vaccines Explained
From Virus to Vaccine: The Science Behind HPV Prevention
HPV Vaccine Development Timeline: From Lab Research to Clinical Application
Ready-to-Use HPV Antibodies
| CAT | Product Name | Target | Type | Price |
|---|---|---|---|---|
| VAnt-Wyb178 | HPV Monoclonal Antibody (E7, IgG2a, 3.3 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb179 | HPV Monoclonal Antibody (E7, IgG1, 3.8 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb180 | HPV Monoclonal Antibody (E7, IgG2a, 3.4 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb181 | HPV Monoclonal Antibody (E6, IgG1-κ, 4.8 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb182 | HPV Monoclonal Antibody (E6, IgG1-κ, 1.01 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb183 | HPV Monoclonal Antibody (L1, IgG1, 3.0 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb184 | HPV Monoclonal Antibody (L1, IgG2a-κ, 4.5 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb185 | HPV Monoclonal Antibody (L1, IgG2a-κ, 3.3 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb186 | HPV Monoclonal Antibody (E7, IgG2b, 4.3 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb187 | HPV Monoclonal Antibody (E7, IgG2a, 4.4 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb188 | HPV Monoclonal Antibody (E7, IgG1, 3.0 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb189 | HPV Monoclonal Antibody (E7, IgG2a, 5.1 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb190 | HPV Monoclonal Antibody (E7, IgG2a, 4.7 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb191 | HPV Monoclonal Antibody (Clone: VA-1870H, IgG1-κ, 1 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb192 | HPV Monoclonal Antibody (Clone: VA-187H, IgG1-κ, 1 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb193 | HPV Monoclonal Antibody (Clone: VA-187H, IgG1, 7.4 mg/ML) | Antibody | Inquiry |
Browse our HPV Antigen Products
→Human Papillomavirus Antigens
Need a custom solution? If our off-the-shelf products aren't a perfect fit, we can create one for you. Contact us to design a product that precisely matches your experimental demands.
References
- Jing, K., et al. "Docosahexaenoic acid induces the degradation of HPV E6/E7 oncoproteins by activating the ubiquitin–proteasome system." Cell death & disease 5.11 (2014): e1524-e1524. https://doi.org/10.1038/cddis.2014.477
- Distributed under Open Access license CC BY 4.0, without modification.
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


