HPV Vaccine Development Timeline: From Lab Research to Clinical Application
Introduction: Biological Basis of HPV and Vaccine Rationale
Human Papillomavirus (HPV) comprises over 200 small, non-enveloped DNA viruses that infect epithelial tissues. Classified by pathogenicity, low-risk subtypes cause benign warts, while high-risk variants drive malignant transformations—most notably cervical cancer, along with other anogenital and oropharyngeal cancers.
HPV's carcinogenic mechanism hinges on two viral proteins: E6 and E7. E6 degrades p53, a critical cell cycle regulator, while E7 inactivates the retinoblastoma protein (pRb), enabling uncontrolled cell proliferation. This disruption of cellular homeostasis creates conditions for cancer development, making HPV a global public health priority.
Vaccine development emerged from a key insight: blocking initial infection would halt carcinogenesis entirely. Unlike culturable viruses, HPV couldn't be grown traditionally in labs, but researchers identified the virus's outer capsid protein, L1, as an ideal immunological target due to its ability to induce protective antibodies. This discovery launched a decades-long journey from basic research to clinical breakthrough.
Early Discoveries: Linking HPV to Cancer (1970s–1980s)
The 1970s–1980s reshaped understanding of HPV's role in cancer. Previously, cervical cancer was attributed to environmental or hormonal factors, but pioneering research shifted this paradigm.
Technical advancements were pivotal: electron microscopy visualized HPV particles in clinical samples, while early nucleic acid sequencing identified distinct viral genotypes. These tools revealed specific HPV subtypes consistently present in cervical cancer tissues.
Transformative studies established causality using molecular hybridization, showing high-risk HPV DNA integrated into the host genome in cancerous cells—rarely seen in non-cancerous tissues. This proved persistent high-risk HPV infection wasn't coincidental but necessary for cervical malignancy, validating preventive vaccine potential.
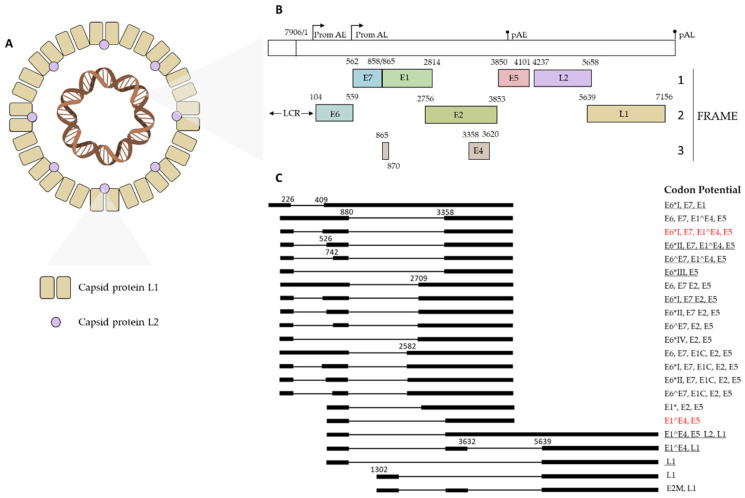 Fig.1 HPV Structure and Genomic Characteristics.1,2
Fig.1 HPV Structure and Genomic Characteristics.1,2
Core Innovation: Virus-Like Particles (1990s)
The 1990s breakthrough—virus-like particles (VLPs)—overcame critical vaccine development hurdles. VLPs are hollow capsid protein structures mimicking intact viruses but lacking replicative genetic material, offering safety and immunogenicity.
VLP formation relies on L1 capsid protein's self-assembling property. When expressed in lab systems, L1 proteins spontaneously form spherical structures identical to natural HPV virions. This structural mimicry triggers robust immune responses without infection risk.
To produce VLPs, researchers used recombinant DNA technology: isolating L1 genes from high-risk subtypes and inserting them into expression vectors (circular DNA facilitating protein production). They tested yeast and insect cell cultures, optimizing systems for proper protein folding—a key to inducing protective immunity.
Immunological studies confirmed VLPs activated B lymphocytes, producing antibodies that neutralized actual HPV particles, preventing host cell infection. This validated VLPs as an effective vaccine platform, advancing preclinical development.
Preclinical Research: Safety and Efficacy Validation (Late 1990s)
Before human trials, extensive preclinical testing evaluated safety and effectiveness, with animal models providing critical insights.
Non-human primates, genetically similar to humans, received experimental VLP vaccines. Studies showed VLPs induced high levels of neutralizing antibodies—proteins blocking viral infection. Subsequent challenge experiments, exposing vaccinated animals to HPV, demonstrated significant protection against infection and related cellular changes.
Rigorous safety testing addressed theoretical risks, with toxicological studies ruling out adverse effects from VLPs or components. Crucially, tests confirmed VLPs (lacking viral DNA) couldn't induce cell transformations or tumor growth, despite HPV's oncogenic reputation.
Dose-ranging studies identified the minimum effective VLP antigen amount generating sufficient antibodies, balancing immunogenicity, safety, and production efficiency—laying scientific groundwork for human trials.
Clinical Trials: Translating Lab Findings (Early 2000s)
Human trials progressed through three phases, expanding participant groups to evaluate safety, dosing, and infection/disease prevention.
Phase I trials in small healthy volunteer groups tested safety and immunogenicity, monitoring adverse reactions and antibody production. Results confirmed tolerability and robust antibody responses matching animal models.
Phase II trials enlarged participant pools and added placebo controls, tracking both antibodies and HPV infection evidence. Data showed vaccinated individuals had significantly lower persistent infection rates with targeted subtypes versus placebo groups, refining dosing schedules (number of injections and intervals).
Phase III trials—the largest and most definitive—involved tens of thousands across countries, measuring clinical endpoints like persistent HPV infections and precancerous lesions (e.g., cervical intraepithelial neoplasia, CIN). Results showed near-complete protection against targeted high-risk subtypes and associated lesions.
The entire clinical trial process spanned approximately a decade, reflecting rigorous standards ensuring safety and effectiveness, with researchers addressing long-term protection and population response variations.
Vaccine Evolution: Expanding Protection (2006–Present)
Post-initial development, research focused on multivalent formulations targeting more subtypes, guided by epidemiological data on clinically significant strains.
Early vaccines targeted two high-risk subtypes causing ~70% of cervical cancers. Subsequent formulations expanded coverage by adding subtypes identified in global cancer prevalence studies, ensuring alignment with public health needs.
A key discovery was cross-protection: vaccines induced antibodies sometimes neutralizing related non-targeted subtypes. This variable cross-reactivity enhanced public health impact beyond primary targets.
Long-term immunity studies tracked vaccinated individuals for over a decade, showing stable protective antibody levels without boosters. This durability stems from memory B cells persisting in the immune system, rapidly producing antibodies upon re-exposure.
Biological Insights: Guiding Principles
HPV vaccine development illustrates how basic science drives medical innovation, with key principles emerging:
Foundational virology research was critical. Understanding HPV's structure, life cycle, and carcinogenic mechanisms identified L1 as a target—applying basic biology to vaccine design.
Interdisciplinary collaboration was essential. Virologists, molecular biologists, immunologists, and epidemiologists collaborated on challenges from VLP design to subtype selection, with epidemiological data directly shaping vaccine composition.
Future directions include broader-spectrum vaccines targeting more high-risk subtypes (vital for regions with diverse HPV patterns) and enhancing mucosal immunity—boosting antibody production at infection entry points to improve prevention.
Accelerate Your HPV Vaccine Research with Creative Biolabs' Expert Design Services
In the competitive and critical field of human papillomavirus (HPV) research, speed and efficacy are paramount. To empower researchers and pharmaceutical companies in this vital work, Creative Biolabs is proud to offer its comprehensive and customizable HPV vaccine design services. By leveraging our state-of-the-art platforms and seasoned scientific expertise, we can significantly reduce research and development timelines, helping you bring your vaccine candidates to the next stage with confidence.
Our end-to-end vaccine development solutions are tailored to meet the specific needs of each project. We offer a diverse array of advanced technologies for HPV vaccine design, including:
- Subunit Vaccine Design: Precisely targeting immunogenic components for a safe and effective response.
- Virus-Like Particle (VLP) based Vaccine Design: Mimicking the natural structure of HPV to elicit a robust and durable immune reaction.
- Viral Vector and DNA/RNA Vaccine Design: Utilizing cutting-edge genetic engineering to optimize antigen presentation and immunogenicity.
At Creative Biolabs, we understand that every research project is unique. Our team of dedicated specialists will work closely with you to develop a strategic and efficient pathway for your HPV vaccine candidate. From initial concept and antigen design to immunogenicity prediction and preclinical assessment, we are your trusted partner in accelerating the fight against HPV.
If you want to learn more about the HPV vaccine, please refer to:
The Molecular Architecture of HPV: Decoding the L1 Capsid Protein
Beyond Prevention: The Science of Therapeutic HPV Vaccines Explained
From Virus to Vaccine: The Science Behind HPV Prevention
HPV E6/E7 Oncoproteins: Key in Therapeutic Vaccine Development
Frequently Asked Questions
Q: Why doesn't the HPV vaccine contain viral DNA?
A: It uses VLPs—capsid proteins without DNA. This design avoids replication/infection risk while triggering antibodies cross-reacting with actual HPV, ensuring safety and immunogenicity.
Q: Biological differences between multi-subtype vaccines?
A: More subtypes mean broader antibody coverage. All protect against common high-risk strains, but expanded formulations add coverage for additional cancer-associated subtypes, reducing overall disease risk without strengthening individual subtype immunity.
Q: How to determine vaccine protection?
A: Blood tests measure antibody levels (higher titers indicate stronger protection), but aren't routine—clinical studies confirm proper vaccination induces sufficient levels. Population data showing reduced infection/disease rates provides definitive effectiveness evidence.
Q: Biological basis for male vaccination?
A: HPV causes cancers in both sexes (anogenital, oropharyngeal). Vaccination induces antibodies preventing infection, reducing male cancer risk and transmission to partners. Mucosal immune responses protect infection entry points, benefiting individuals and public health.
HPV vaccines exemplify translating biological research into cancer prevention tools. By targeting viral structures and leveraging immune defenses, they transform global health outcomes, with future innovations promising enhanced protection against HPV-related diseases.
Ready-to-Use HPV Antibodies
| CAT | Product Name | Target | Type | Price |
|---|---|---|---|---|
| VAnt-Wyb178 | HPV Monoclonal Antibody (E7, IgG2a, 3.3 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb179 | HPV Monoclonal Antibody (E7, IgG1, 3.8 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb180 | HPV Monoclonal Antibody (E7, IgG2a, 3.4 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb181 | HPV Monoclonal Antibody (E6, IgG1-κ, 4.8 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb182 | HPV Monoclonal Antibody (E6, IgG1-κ, 1.01 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb183 | HPV Monoclonal Antibody (L1, IgG1, 3.0 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb184 | HPV Monoclonal Antibody (L1, IgG2a-κ, 4.5 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb185 | HPV Monoclonal Antibody (L1, IgG2a-κ, 3.3 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb186 | HPV Monoclonal Antibody (E7, IgG2b, 4.3 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb187 | HPV Monoclonal Antibody (E7, IgG2a, 4.4 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb188 | HPV Monoclonal Antibody (E7, IgG1, 3.0 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb189 | HPV Monoclonal Antibody (E7, IgG2a, 5.1 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb190 | HPV Monoclonal Antibody (E7, IgG2a, 4.7 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb191 | HPV Monoclonal Antibody (Clone: VA-1870H, IgG1-κ, 1 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb192 | HPV Monoclonal Antibody (Clone: VA-187H, IgG1-κ, 1 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
| VAnt-Wyb193 | HPV Monoclonal Antibody (Clone: VA-187H, IgG1, 7.4 mg/ML) | Human Papillomavirus | Antibody | Inquiry |
Browse our HPV Antigen Products
→Human Papillomavirus Antigens
Need a custom solution? If our off-the-shelf products aren't a perfect fit, we can create one for you. Contact us to design a product that precisely matches your experimental demands.
References
- Folliero, Veronica, et al. "Epigenetic and genetic keys to fight HPV-related cancers." Cancers 15.23 (2023): 5583. https://doi.org/10.3390/cancers15235583
- Distributed under Open Access license CC BY 4.0, without modification.
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


