The urgent need for effective antibacterial strategies, particularly for immunocompromised patients post-CAR T-cell therapy, is paramount. Creative Biolabs offers a bacterial infection assay service to address the challenges of long drug development cycles and complex preclinical testing by accelerating anti-infective drug discovery, delivering precise efficacy data, and developing potent antibacterial agents through advanced microbiological techniques, robust screening platforms, and innovative infection models.
The landscape of modern medicine, particularly in advanced therapies like CAR T-cell therapy, has introduced new complexities in managing patient health. Patients undergoing CAR T-cell therapy often experience significant immunosuppression, making them highly susceptible to severe bacterial infections. These infections represent a major cause of morbidity and mortality post-treatment, frequently complicating recovery and impacting overall therapeutic outcomes. Therefore, there is a critical and urgent need for robust bacterial infection management strategies to understand the baseline susceptibility of these patients, monitor for emerging resistance, and develop effective prophylactic and therapeutic antibacterial strategies specifically tailored to this vulnerable population. This is indispensable for improving patient safety and the success of innovative immunotherapies.
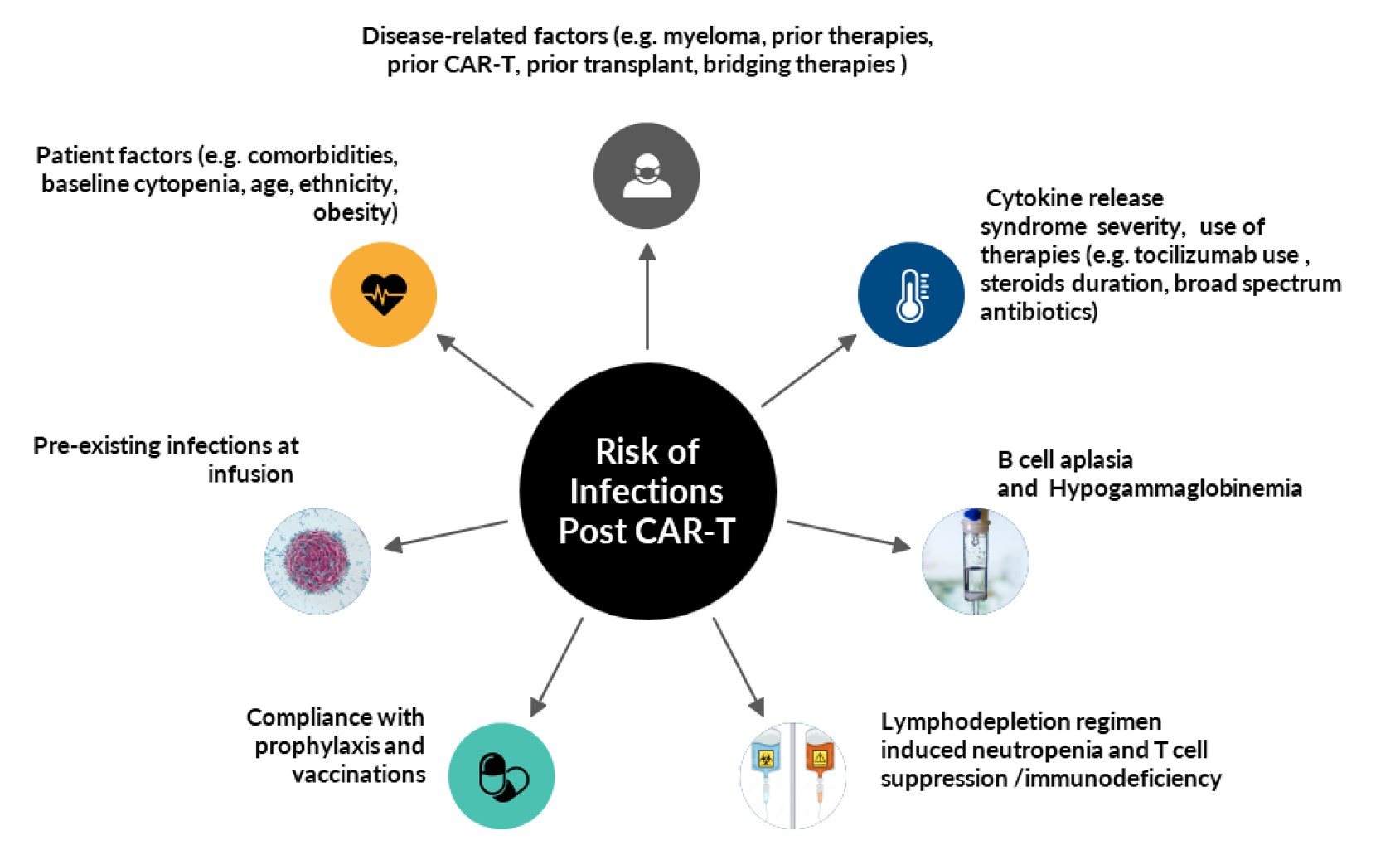 Fig.1 Risk factors for post-CAR-T infection.1
Fig.1 Risk factors for post-CAR-T infection.1
Creative Biolabs offers a comprehensive bacterial infection assay service designed to provide specific deliverables, actionable solutions, and key problem-solving capabilities tailored to your anti-infective research and development needs. We deliver precise data on compound efficacy, bacterial susceptibility, and host-pathogen dynamics, enabling informed decision-making throughout your project lifecycle. Our service follows a meticulously designed workflow, ensuring comprehensive and reliable results. Typically, the timeline for our assay ranges from 4-12 weeks, depending on the complexity of the bacterial strains, the number of compounds, and the chosen assay models.
This initial phase involves an in-depth discussion with our expert team to understand your specific research objectives, target pathogens, compound characteristics, and desired endpoints. We will collaboratively define the scope of your project, select the most appropriate in vitro and/or in vivo infection models, and establish a detailed experimental plan.
Our microbiologists will culture and prepare your specified bacterial strains under optimal conditions. This includes verifying strain purity, assessing viability, and preparing standardized inoculums for various assays. For specific projects, we can also perform preliminary characterization of resistance profiles or virulence factors.
This core stage involves the precise execution of selected bacterial infection assays.
Following assay execution, our bioinformatics and microbiology specialists will meticulously process the raw data. This includes statistical analysis, normalization, and graphical representation of results. We apply rigorous analytical methods to ensure the quality.
The final phase involves the delivery of a detailed, comprehensive report summarizing all experimental procedures, raw data, analyzed results, and their interpretation. Our team will provide an expert consultation to discuss the findings, their implications for your project, and recommend next steps for further research or development.
Through our Bacterial Infection Assay Service, a leading oncology research institute successfully identified a novel prophylactic antibiotic regimen specifically effective against common bacterial infections in CAR T-cell therapy patients, significantly reducing post-treatment complications and improving patient outcomes.
After utilizing Creative Biolabs' Bacterial Infection Assay Service, this biopharmaceutical company successfully validated the efficacy of their new antimicrobial agent against a multi-drug-resistant Pseudomonas aeruginosa strain isolated from a CAR T-cell recipient, demonstrating its potential for managing critical infections in immunocompromised patients.
Q1: What types of bacterial strains can Creative Biolabs work with for infection assays?
A1: We have extensive experience working with a wide range of bacterial strains, including common pathogens, multi-drug resistant (MDR) strains, and fastidious organisms. We can also accommodate client-provided strains. Please discuss your specific strain requirements during the initial consultation, and we'll confirm our capabilities.
Q2: Can Creative Biolabs develop custom infection models for unique research challenges?
A2: Absolutely. While we offer a comprehensive suite of established models, our strength lies in our ability to develop and validate custom in vitro and in vivo infection models tailored to your specific research needs, including novel host-pathogen interactions or unique disease manifestations. Let's discuss your custom requirements!
In addition to the bacterial infection assay, we also provide a series of infection assays for many other microorganisms that threaten patients after CART therapy treatment. Our services include:
Creative Biolabs is your trusted partner in advancing anti-infective research. Our bacterial infection assay service provides the critical data and insights needed to accelerate your drug discovery programs, from initial compound screening to preclinical validation. If you want to know more about this assay, please feel free to contact us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION