Patients undergoing advanced cellular immunotherapies, such as those involving CD19-targeted chimeric antigen receptor (CAR)–modified T cells, B-cell maturation antigen (BCMA)–targeted CAR T cells, and natural killer cells, face a heightened risk of severe respiratory viral infections (RVIs), leading to increased morbidity and mortality. Creative Biolabs provides a respiratory viral infection assay service that aims to address critical challenges in this field, such as long diagnostic cycles, difficulties in identifying precise viral strains, complexities in evaluating antiviral efficacy, and the intricate demands of clinical trials for respiratory diseases.
Respiratory viral infections, including influenza, respiratory syncytial virus (RSV), and SARS-CoV-2, represent a persistent global health challenge, causing significant morbidity and mortality across all demographics, particularly in immunocompromised individuals. The dynamic nature of these viruses, characterized by rapid evolution and the emergence of new strains, necessitates continuous innovation in diagnostic and research tools. Precise and prompt identification of particular viral viruses is essential for public health initiatives, clinical care, and co-infection distinction. Furthermore, robust assay services are indispensable for the discovery and development of novel antiviral therapeutics and vaccines, enabling precise evaluation of viral kinetics and host immune responses.
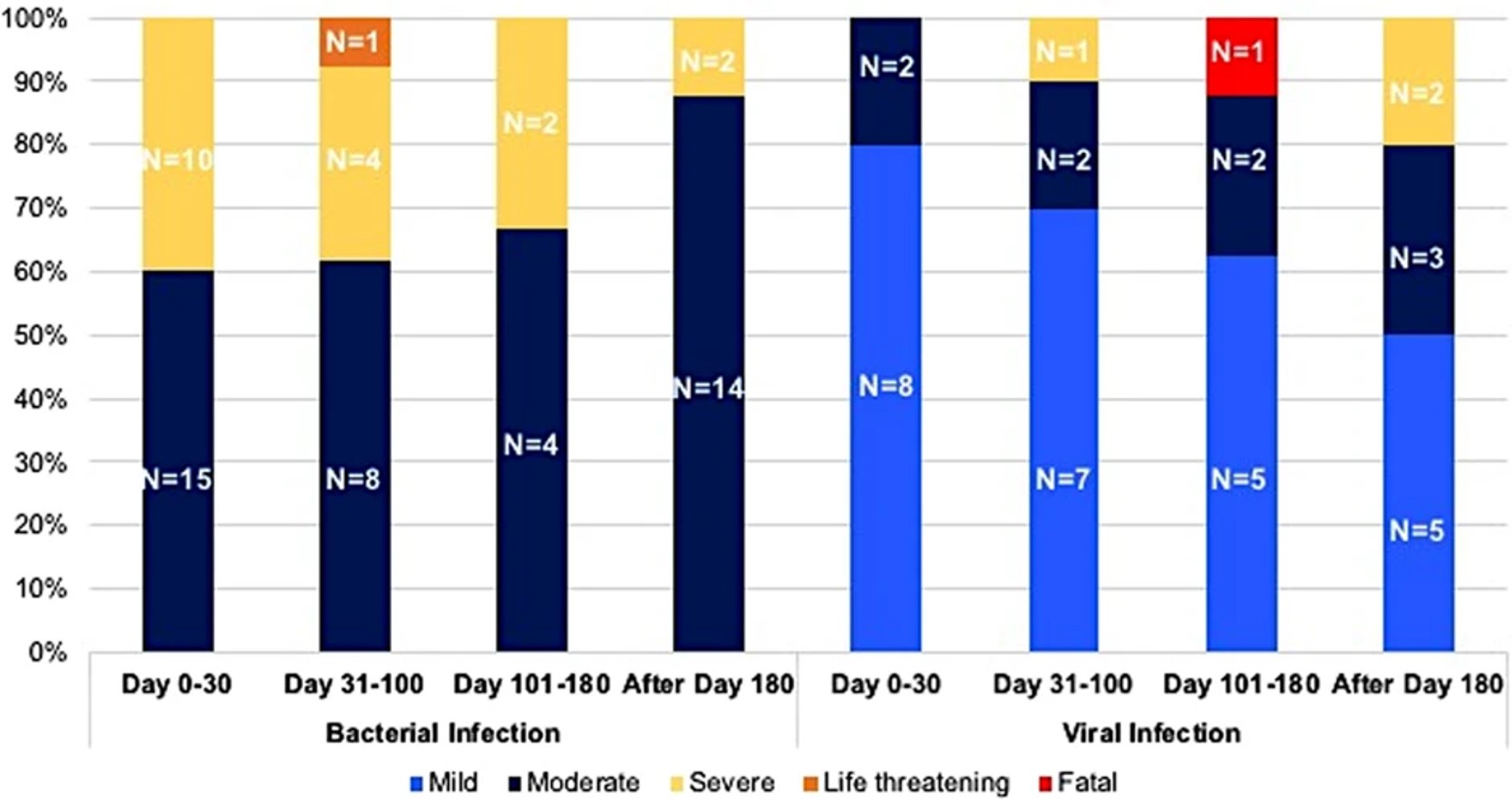 Fig.1 Infections following CD19 CAR T cell treatment.1
Fig.1 Infections following CD19 CAR T cell treatment.1
Creative Biolabs' respiratory viral infection assay service provides comprehensive solutions designed to meet the rigorous demands of antiviral drug discovery, vaccine development, and clinical research. Our workflow is meticulously designed to ensure high-quality results and clear, actionable insights, providing a streamlined path for your research and development needs. We deliver precise, reliable data crucial for advancing your projects, from early-stage screening to preclinical evaluation.
We begin with an in-depth discussion to understand your specific research objectives, target viruses, sample types, and desired assay requirements. This collaborative approach ensures the development of a tailored assay strategy.
Rigorous preparation of your provided samples is paramount. This includes nucleic acid extraction, viral particle quantification, and comprehensive sample integrity checks to ensure optimal assay performance.
Our expert team performs the chosen assays using state-of-the-art platforms. This may include quantitative PCR (qPCR) for viral load, ELISA for antibody detection, cytopathic effect (CPE) assays, plaque assays for viral infectivity, or neutralization assays for antiviral efficacy.
Raw data undergoes thorough statistical analysis, data visualization, and comparison against established controls and standards. Our bioinformaticians and virologists interpret the results in the context of your project goals.
We provide a comprehensive, detailed experimental report summarizing methodologies, raw data, analyzed results, and our expert interpretations. A follow-up consultation is arranged to discuss the findings and recommend next steps.
Through our Respiratory Viral Infection Assay Service, a leading pharmaceutical company successfully identified multiple novel antiviral compounds against influenza within a significantly reduced timeframe, accelerating their preclinical development. Our high-throughput screening assays enabled rapid evaluation of compound libraries, leading to the prioritization of promising candidates.
After using our Respiratory Viral Infection Assay Service, a prominent academic research institution successfully completed a large-scale seroprevalence study for RSV, providing critical epidemiological data for public health initiatives. Our robust immunoassay platforms ensured accurate and reproducible results across thousands of samples, contributing to a deeper understanding of RSV transmission dynamics.
Q1: What types of respiratory viruses can your assays detect?
A1: Our service is designed to detect a broad spectrum of respiratory viruses, including but not limited to Influenza A/B, respiratory syncytial virus (RSV), human metapneumovirus (hMPV), parainfluenza viruses (PIV), rhinoviruses, adenoviruses, and various coronaviruses including SARS-CoV-2. If you have a specific viral target, please inquire, and we can discuss custom assay development.
Q2: Can your service be adapted for high-throughput screening of antiviral compounds?
A2: Our systems are extremely versatile for high-throughput screening. We can develop customized assays to screen large libraries of compounds for antiviral activity against your target respiratory virus, providing critical data for lead compound identification and optimization.
After CAR-T cell therapy, patients can be vulnerable to a range of infections beyond just respiratory viruses. We offer comprehensive infectious disease assays targeting a wide array of microorganisms that pose a risk to these immunocompromised patients. Our services include:
For more details about our respiratory viral infection assay service, please don't hesitate to get in touch with us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION