Are you currently facing challenges in evaluating antiviral efficacy against latent HSV, particularly in the context of immunosuppressive therapies like CAR T-cell treatment, or difficulty in assessing reactivation mechanisms? Creative Biolabs' HSV reactivation assay service helps you accelerate antiviral drug discovery, accurately evaluate drug candidates, and understand HSV latency and reactivation through advanced cell-based assay platforms and robust viral detection methods.
Herpes Simplex Virus (HSV) establishes lifelong latency, causing recurrent disease that current antivirals cannot eliminate. This presents a significant challenge, particularly for patients undergoing advanced immunosuppressive treatments like CAR T-cell therapy. Such therapies create a profound immunosuppressive microenvironment, making patients highly susceptible to opportunistic infections, including common and serious HSV reactivation. Viral infections, including HSV, significantly contribute to morbidity and mortality in immunocompromised individuals. The persistent clinical burden and the critical need for improved patient safety necessitate advanced HSV reactivation assays. These assays are vital for identifying novel therapeutic targets and evaluating drug candidates to prevent or reduce reactivation, ultimately improving patient outcomes post-CAR T-cell therapy.
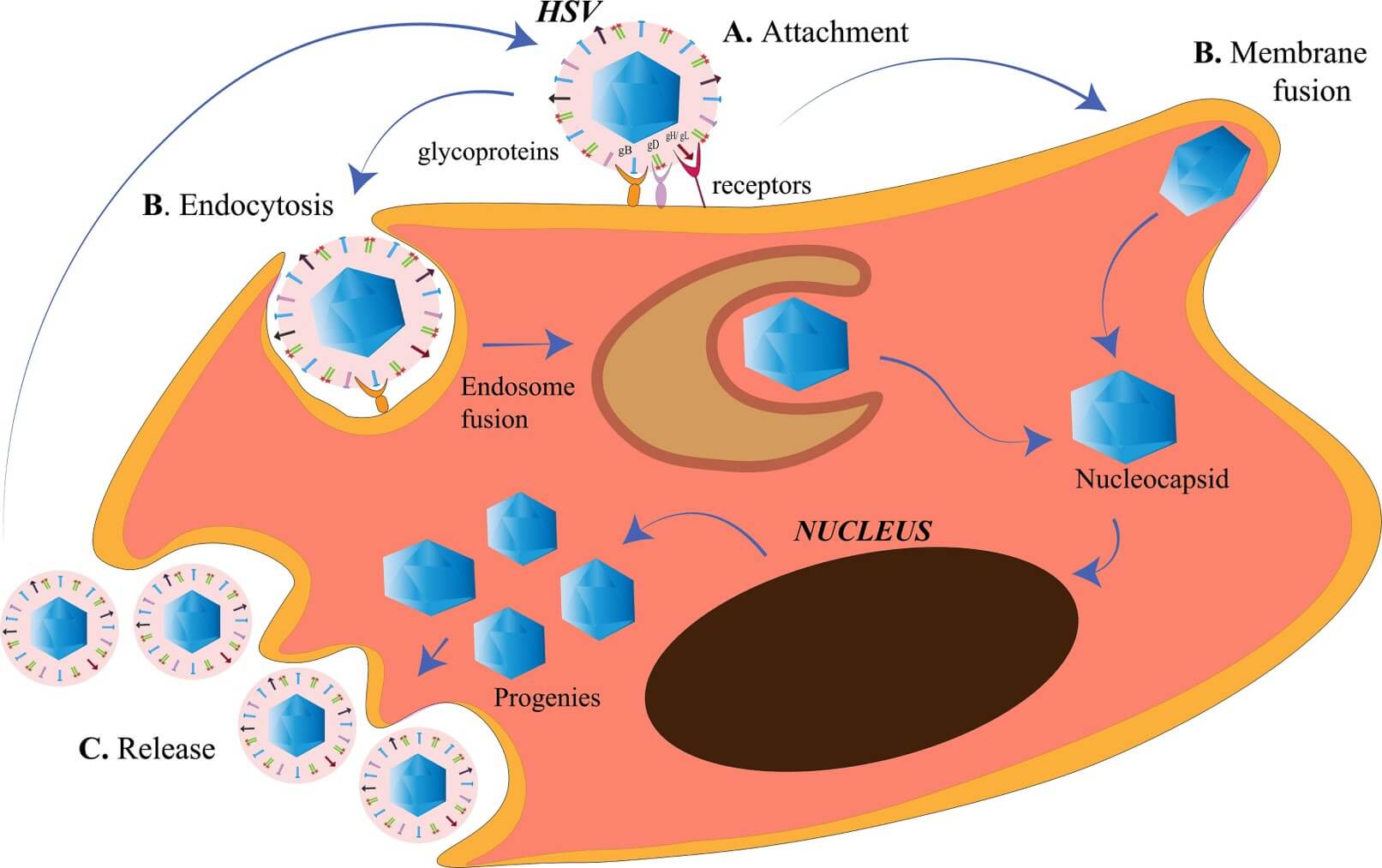 Fig.1 The process of HSV entry and release.1
Fig.1 The process of HSV entry and release.1
Creative Biolabs' HSV reactivation assay service provides comprehensive solutions for evaluating compounds and understanding the mechanisms governing HSV latency and reactivation. Our service is designed to deliver precise, reliable data that accelerates your antiviral drug discovery and development efforts. You can expect detailed insights into compound efficacy against latent virus, identification of molecules capable of inhibiting reactivation, and a clearer understanding of viral pathogenesis, crucial for managing infections in immunosuppressed patients.
We establish and optimize appropriate in vitro latency models, typically using neuronal or neuronal-like cell lines (e.g., human neuroblastoma cells, primary sensory neurons) that faithfully recapitulate HSV latency. This involves culturing and preparing cells under conditions conducive to establishing a quiescent viral state.
Cells are infected with HSV at a low multiplicity of infection (MOI) and maintained under specific conditions (e.g., low temperature, chemical inhibitors, or neuronal co-culture) to promote the establishment of a latent infection, where viral gene expression is minimal and no productive replication occurs.
Latent HSV is induced to reactivate using various physiological or chemical stimuli known to trigger reactivation in vivo (e.g., stress hormones, UV irradiation, histone deacetylase inhibitors, or specific cytokines). This step ensures the model accurately reflects in vivo reactivation triggers, including those relevant to immunosuppression.
Raw data undergoes thorough statistical analysis, data visualization, and comparison against established controls and standards. Our bioinformaticians and virologists interpret the results in the context of your project goals.
Post-reactivation, we quantify viral replication and gene expression using highly sensitive methods such as quantitative PCR (qPCR) for viral genome copies, reverse transcription qPCR (RT-qPCR) for lytic gene expression, and immunofluorescence or Western blot for viral protein detection. This provides a comprehensive picture of reactivation levels.
The collected data undergoes rigorous statistical analysis. We provide detailed reports, including dose-response curves, statistical significance of compound effects, and comparative analyses against controls. Our expert scientists interpret the findings to provide actionable insights for your project, including implications for patient management in immunosuppressive contexts.
Review One----Enhanced Efficacy Screening
Using Creative Biolabs' HSV Reactivation Assay Service in our research has significantly improved our ability to screen compounds for anti-reactivation potential, providing clearer efficacy profiles compared to standard antiviral assays. The detailed data allowed us to prioritize our lead candidates more effectively, especially for our projects targeting viral control in immunosuppressed patients.
----2024-03-15, Dr. J***s M
Review Two----Streamlined Compound Evaluation
The comprehensive workflow and clear deliverables from Creative Biolabs' HSV Reactivation Assay Service streamlined our compound evaluation process. We gained critical insights into the anti-reactivation activity of our novel molecules, which has been instrumental in guiding our preclinical development for preventing post-transplant viral complications. Their team's scientific support was exceptional.
----2025-01-10, Dr. A***a P
Q1: What types of HSV strains can be tested with this assay?
A1: Our HSV Reactivation Assay Service is highly versatile and can accommodate a wide range of HSV-1 and HSV-2 strains, including common laboratory strains, drug-resistant variants, and specific clinical isolates. We work closely with you to select the most appropriate strain for your research objectives.
Q2: Can this service be used to evaluate both small molecules and biologics?
A2: Yes, our assay platform is adaptable for evaluating various types of compounds, including small molecules, peptides, antibodies, and other biologics. We can optimize the assay conditions to suit the specific characteristics of your test compounds, making it suitable for a broad range of antiviral development efforts.
Q3: What materials do I need to provide to start a project?
A3: To initiate your project, we typically require specific HSV-1 or HSV-2 viral strains (e.g., clinical isolates), your antiviral drug candidates or compounds for screening, and any relevant cell lines that mimic neuronal latency or are critical to your research.
Following CAR-T cell treatment, individuals may be susceptible to infections other than HSV. We provide comprehensive infectious disease tests that target a diverse range of organisms that represent a danger to immunocompromised individuals. Our services include:
At Creative Biolabs, we are dedicated to advancing antiviral research through our cutting-edge HSV Reactivation Assay Service. Our expertise and comprehensive solutions are designed to accelerate your projects and bring effective antiviral therapies closer to patients, especially those in vulnerable, immunosuppressed states following treatments like CAR T-cell therapy. If you want to learn more about this service, please feel free to reach out to us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION