Addressing invasive fungal infections in immunocompromised patients, particularly after CAR-T therapy, is a significant clinical challenge. Delayed diagnosis, the emergence of drug-resistant strains, and the need for rapid antifungal efficacy evaluation often hinder progress. Our fungal infection assay service accelerates antifungal drug discovery, obtains accurate and rapid fungal identification, evaluates antifungal agent potency, and overcomes resistance challenges through advanced assay platforms, high-throughput screening, and innovative detection methods tailored for vulnerable patient populations.
Patients undergoing advanced immunotherapies like CAR-T cell therapy often develop a profoundly immunosuppressive microenvironment, leaving them highly susceptible to severe and often fatal invasive fungal infections (IFIs). The incidence of IFIs in this vulnerable population is a significant concern, as highlighted by recent studies. Current diagnostic limitations, including slow turnaround times and lack of specificity, exacerbate the challenge, leading to delayed interventions and increased mortality. Therefore, the urgent development of rapid, sensitive, and specific fungal infection assays is paramount to enable early detection, guide targeted antifungal therapy, and effectively combat the escalating threat of antifungal resistance in these critically ill patients. This is crucial for improving patient outcomes and accelerating the discovery of novel, effective antifungal agents.
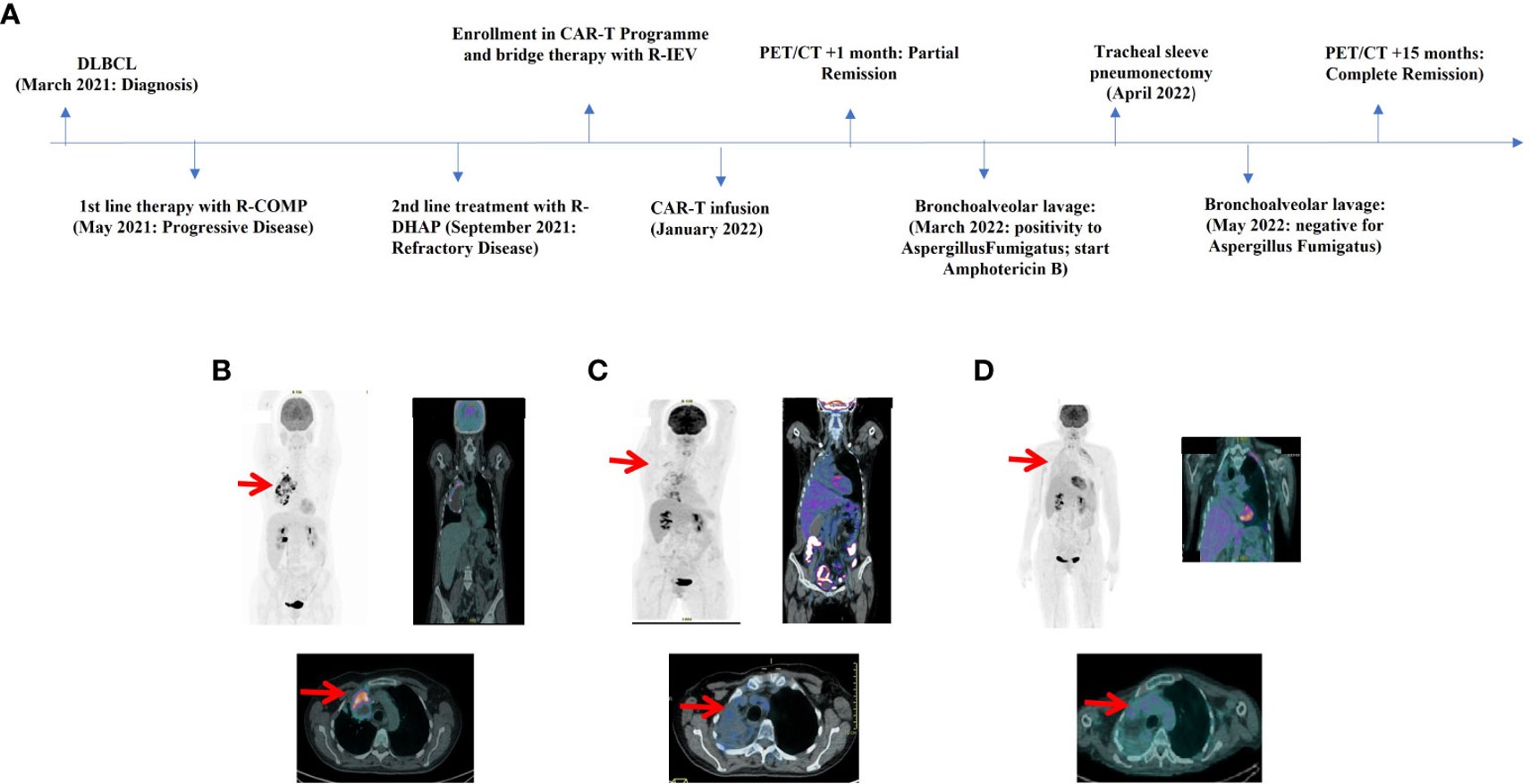 Fig.1 Timelines of the treatments administered and their corresponding results.1
Fig.1 Timelines of the treatments administered and their corresponding results.1
Creative Biolabs' fungal infection assay service provides comprehensive solutions for the identification, characterization, and susceptibility testing of fungal pathogens, along with robust platforms for antifungal drug screening and mechanism of action studies, specifically addressing the complexities of infections in immunocompromised settings. Our service operates through a meticulously designed workflow, ensuring efficiency and reliability from initial consultation to final report. We deliver precise and actionable data to advance your research and development efforts. Projects typically range from 2 to 6 weeks, depending on complexity, and are underpinned by our stringent quality control measures, guaranteeing accurate and reproducible results.
Initial assessment and processing of client-provided samples, with particular attention to the unique characteristics of clinical samples from immunocompromised patients, to ensure suitability for downstream assays. This includes fungal culture, DNA/RNA extraction, or compound solubilization.
Tailoring the assay protocol to specific project requirements, including selection of appropriate fungal strains (e.g., clinical isolates from CAR-T patients), assay formats (e.g., MIC, time-kill, biofilm, host-pathogen interaction models relevant to immunosuppression), and highly sensitive detection methods.
Performing the selected assays using advanced platforms, such as automated liquid handlers for large-scale compound screening or molecular diagnostics for rapid pathogen identification, prioritizing methods that offer speed and sensitivity crucial for immunocompromised patient management.
Collection of quantitative data from the assays (e.g., OD readings, fluorescence intensity, gene expression levels) followed by rigorous statistical analysis and interpretation, with expert insights into the implications for immunocompromised patient populations
Compilation of all experimental details, raw data, analyzed results, and conclusions into a detailed, easy-to-understand report. This includes methodology, quality control data, and interpretation of findings, providing actionable insights for clinical translation or further drug development.
Q1: What types of fungal species can your assays detect and characterize, especially those relevant to immunocompromised patients?
A1: Our service is highly versatile and can detect and characterize a broad spectrum of pathogenic fungi, including common species like Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans, which frequently cause infections in immunocompromised individuals. We also specialize in emerging multidrug-resistant strains such as Candida auris, often found in high-risk patient populations. We can customize assays for specific or less common fungal pathogens based on your project needs, including those isolated from CAR-T patients. Feel free to contact us with your specific requirements!
Q2: Can your service help us screen novel antifungal compounds specifically for efficacy in immunosuppressed hosts?
A2: Of course. Our Fungal Infection Assay Service includes robust platforms for high-throughput screening of novel antifungal compounds. We can evaluate their potency, determine MIC values, assess fungicidal or fungistatic activity, and investigate their mechanism of action against various fungal targets. Crucially, we can design custom screening cascades and in vitro models that mimic the immunosuppressive microenvironment, allowing you to assess compound efficacy under conditions relevant to CAR-T therapy patients or other immunocompromised individuals.
Q3: We are working with a unique fungal strain isolated from a CAR-T patient; can you develop a custom assay for it?
A3: Yes, customization is a cornerstone of our service. We specialize in developing bespoke assays for unique fungal strains, especially those isolated from specific patient populations like CAR-T recipients, or for investigating complex host-pathogen interactions under immunosuppressive conditions. Our scientific team will collaborate closely with you to design and optimize a tailored assay protocol that precisely meets your project's objectives, ensuring relevance and accuracy for your specific research focus.
Q4: What specific preparation is needed for patient samples or fungal isolates before submission?
A4: For patient samples (e.g., blood, CSF, tissue biopsies), proper collection and transport are crucial to maintain sample integrity. We recommend following standard clinical guidelines for aseptic collection and immediate refrigeration or freezing, depending on the sample type and target analyte. For fungal isolates, please provide pure cultures in a viable state, preferably on appropriate agar slants or cryopreserved. Detailed shipping instructions and any specific pre-treatment requirements will be provided upon project initiation to ensure optimal sample quality for our assays.
We also offer a comprehensive suite of services for monitoring other microorganisms.
Creative Biolabs is your trusted partner in advancing antifungal research and drug development, especially for the critical needs of immunocompromised patients. Our fungal infection assay service delivers precise, reliable, and timely data, empowering you to make informed decisions and accelerate your projects. For more details about our service, please feel free to get in touch with us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION