Chimeric Antigen Receptor (CAR) T-cell therapy has revolutionized the treatment of various hematologic malignancies. However, it comes with unique toxicities, including a heightened risk of infections. Among these, Varicella-Zoster Virus (VZV) reactivation is a common and potentially serious complication after CAR T-cell therapy. Creative Biolabs offers the VZV reactivation assay service to mitigate clinical risks associated with advanced biotherapies and streamlines viral safety assessments through advanced cell-based assays and highly sensitive detection platforms.
Gene therapies, particularly CAR T-cell therapy, induce profound immunosuppression, making patients highly susceptible to opportunistic infections, including Varicella-Zoster Virus (VZV) reactivation, which endangers patient safety. Clinical evidence shows this immunosuppressive state significantly elevates VZV reactivation risk, leading to severe complications like herpes zoster, encephalitis, or disseminated disease. Therefore, developing and applying highly sensitive VZV reactivation assays is paramount. These assays are indispensable for timely detection, enabling prompt antiviral intervention, safeguarding patient health, and ensuring the success of these transformative biopharmaceutical treatments.
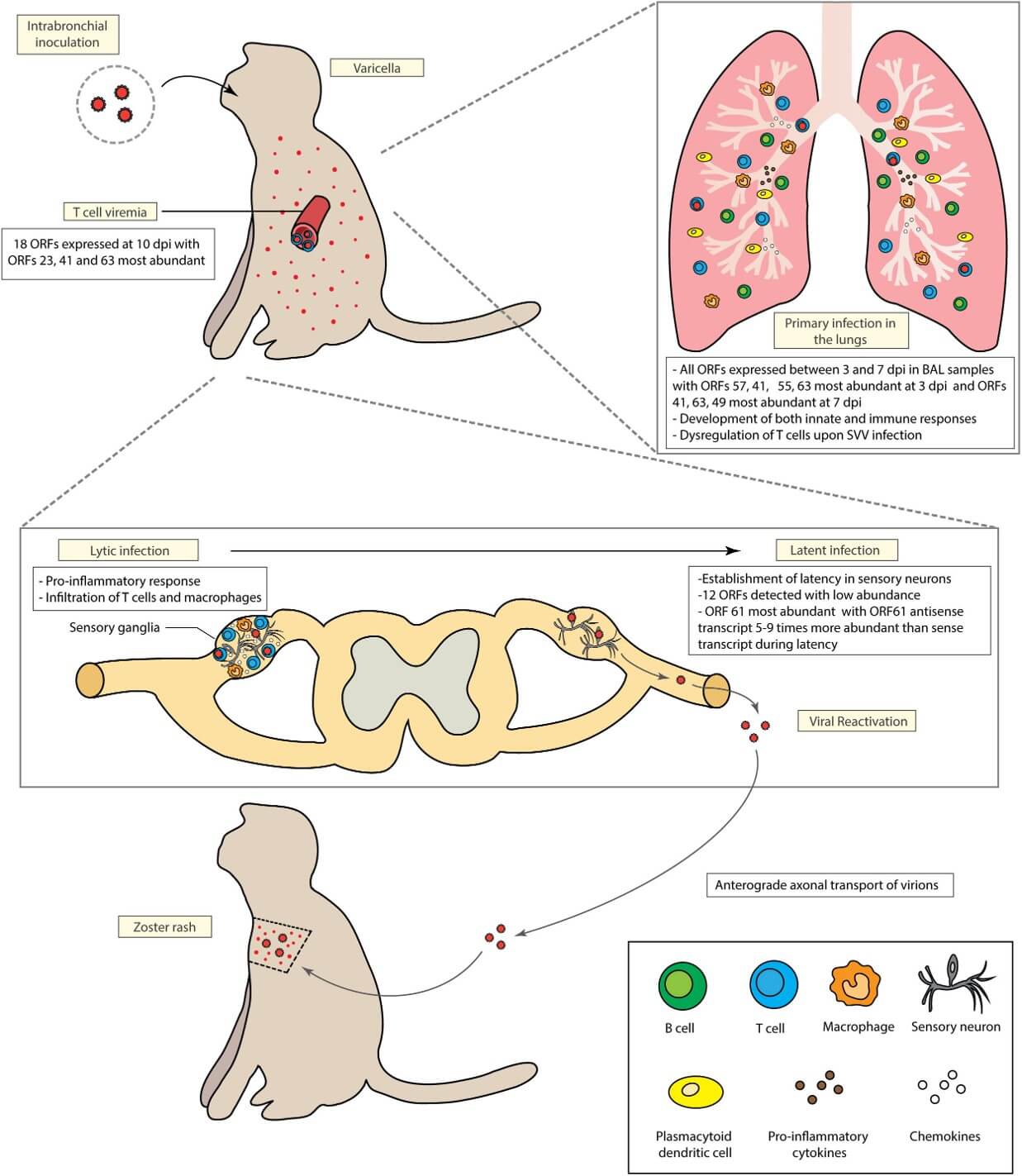 Fig.1 Pathophysiological model of Simian Varicella Virus (SVV) in rhesus macaques after intrabronchial injection.1
Fig.1 Pathophysiological model of Simian Varicella Virus (SVV) in rhesus macaques after intrabronchial injection.1
Creative Biolabs' VZV reactivation assay service offers comprehensive solutions for accurate VZV detection and quantification, critical for viral safety in advanced therapeutic development. Our service begins with sample preparation and clinical data review, progressing to cell culture and co-culture setups for VZV induction and monitoring. We utilize highly sensitive qPCR for viral load and immunological assays (ELISA/Flow Cytometry) for host-virus insights. This workflow provides precise VZV reactivation data, supporting robust risk assessment, preventing complications, and supplying essential information for regulatory submissions (IND/BLA). Projects typically take 6-10 weeks, depending on complexity, empowering effective patient monitoring and ensuring biopharmaceutical safety.
Case One
A leading gene therapy company partnered with Creative Biolabs for their VZV Reactivation Assay Service. Through our service, they successfully identified multiple instances of VZV reactivation in their patient cohort undergoing novel CAR T-cell therapy, enabling timely prophylactic intervention and preventing severe clinical complications within 8 weeks. This critical data significantly enhanced their patient safety monitoring protocols, directly addressing the risks of immunosuppression.
Review One----Comprehensive Insights into Immune Status
The comprehensive data provided by Creative Biolabs, including both viral load quantification and immunological markers, greatly facilitated our understanding of the altered host-virus interaction in our gene therapy studies, offering a distinct advantage over standard PCR methods by providing a more complete picture of VZV risk in immunosuppressed individuals
----- 2025-3-25, Ms. S***h
Q1: What types of samples are suitable for the VZV Reactivation Assay Service, especially from immunocompromised patients?
A1: Our service is versatile and can accommodate various sample types, including peripheral blood mononuclear cells (PBMCs), plasma, and cerebrospinal fluid (CSF), depending on your specific research needs and the clinical context of immunosuppression. We recommend discussing your sample type with our experts to ensure optimal results and assay compatibility for these sensitive samples.
Q2: How sensitive is Creative Biolabs' VZV Reactivation Assay compared to traditional methods for detecting VZV in immunosuppressed individuals?
A2: Our assay utilizes advanced molecular and cell-based techniques, offering superior sensitivity and specificity compared to traditional methods. This allows for the detection of even low levels of VZV reactivation, providing critical early insights that are often missed by less sensitive approaches, thus enabling more timely clinical decisions and enhanced patient safety in immunosuppressed populations.
Q3: What specific information or materials are required to initiate the VZV Reactivation Assay Service?
A3: To begin, we require patient-derived samples such as PBMCs, plasma, or CSF, collected and handled under sterile conditions. Additionally, relevant clinical data, including patient history, current treatment regimen (e.g., CAR T-cell therapy), and detailed immunosuppression status, are essential for contextualizing results and assessing patient risk. Any specific assay requirements or desired readouts should also be communicated.
In addition, we also provide a suite of services to monitor other microorganisms, which includes:
At Creative Biolabs, our VZV reactivation assay service provides an essential, high-sensitivity tool for monitoring viral safety in advanced biopharmaceutical development, directly addressing the critical patient safety concerns arising from immunosuppression in treatments like CAR T-cell therapy. If you are interested in our service, please click the link to get in touch with us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION