To addresses long drug development cycles and challenges in antibody development, Creative Biolabs provides a cost-effective bone marrow CD34⁺ derived dendritic cell (DC): next-gen DC generation service to help you accelerate drug discovery and develop highly specific antibodies through advanced recombinant DNA technology and innovative protein engineering techniques. This service ensures the generation of high-quality, functionally potent dendritic cells crucial for robust immune responses.
Dendritic cells (DCs) are critical orchestrators of the immune response, central to activating T-cell and NK cell responses against tumors and pathogens. While monocyte-derived DCs (moDCs) have been widely used, their clinical efficacy in cancer patients has often been limited. Natural DC subsets, particularly CD34⁺-derived conventional DCs (cDC1s) and plasmacytoid DCs (pDCs), offer superior antigen presentation and cross-talk capabilities. However, their scarcity in peripheral blood makes obtaining sufficient numbers for therapeutic applications challenging. Developing efficient protocols for generating these next-gen DCs from bone marrow CD34⁺ hematopoietic stem cells is essential to overcome current limitations in gene therapy and advance robust, targeted immunotherapies.
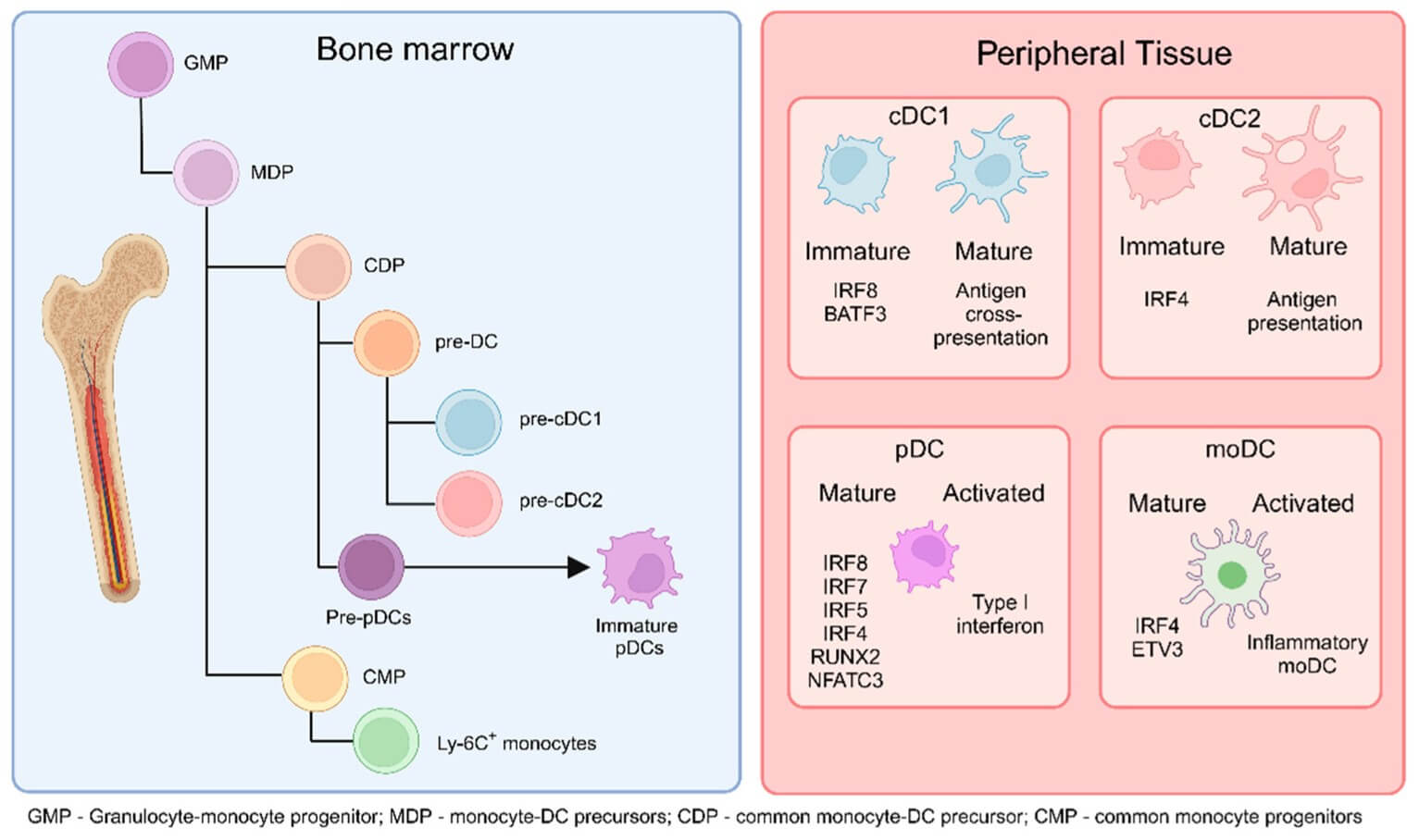 Fig.1 DCs originate from common monocyte-DC precursors (MDPs) in the bone marrow, which are derived from granulocyte-monocyte progenitors.1
Fig.1 DCs originate from common monocyte-DC precursors (MDPs) in the bone marrow, which are derived from granulocyte-monocyte progenitors.1
Creative Biolabs' bone marrow CD34⁺ derived dendritic cell (DC): next-gen DC generation service provides high-quality, functionally potent dendritic cells for your research and therapeutic development. Our service operates on the principle of controlled ex vivo differentiation, guiding multipotent CD34⁺ hematopoietic stem cells into specific, highly effective DC subsets through optimized cytokine cocktails and culture conditions. This approach ensures the generation of DCs with enhanced antigen-presenting capabilities, crucial for advancing vaccine development, cancer immunotherapy, and studies on immune regulation. You can expect well-characterized cells, detailed phenotypic and functional data, and reliable support, all delivered within a typical timeframe of 6 to 10 weeks, accelerating your project timelines with guaranteed high purity, viability, and consistent functional activity.
Highly pure CD34⁺ hematopoietic stem cells are meticulously isolated from the provided bone marrow aspirate or PBMCs using advanced immunomagnetic sorting techniques. This critical initial step ensures a clean and enriched starting population, which is fundamental for optimal differentiation efficiency and the generation of specific DC subsets. The outcome is a highly purified population of CD34⁺ cells, ready for expansion.
The isolated CD34⁺ cells are then placed in optimized culture conditions, utilizing specialized media and a proprietary blend of growth factors. This phase promotes robust expansion of the precursor cells while carefully maintaining their multipotent characteristics. The expected outcome is a significant increase in the total number of CD34⁺ cells, providing a sufficient foundation for subsequent differentiation.
During this pivotal stage, the expanded CD34⁺ cells are carefully guided to differentiate into specific DC subsets, such as conventional DCs (cDCs) or plasmacytoid DCs (pDCs). This is achieved by culturing them in the presence of a precisely formulated cocktail of cytokines, including GM-CSF, IL-4, and Flt3-L. The precise ratios and timing of these factors dictate the lineage commitment, resulting in phenotypically distinct DC populations.
For applications requiring fully functional and immunostimulatory DCs, an optional maturation step is introduced. This involves exposing the differentiated DCs to various stimuli, such as TLR agonists or inflammatory cytokines, to enhance their antigen-presenting capacity, co-stimulatory molecule expression, and T-cell activation potential. This step yields highly activated DCs suitable for therapeutic or advanced research.
Creative Biolabs employs rigorous quality control measures throughout the process. Differentiated DCs undergo comprehensive phenotypic analysis via flow cytometry, assessing surface marker expression (e.g., CD1a, CD11c, HLA-DR, CD83, CD86). Functional characterization includes cytokine secretion assays and antigen presentation assays (e.g., mixed leukocyte reaction). This ensures the cells consistently meet stringent purity, viability, and functional criteria.
Q1: What types of dendritic cells can Creative Biolabs generate from CD34⁺ precursors?
A1: We can generate various functionally distinct DC subsets, including conventional dendritic cells type 1 (cDC1s), conventional dendritic cells type 2 (cDC2s), and plasmacytoid dendritic cells (pDCs), meticulously tailored to your specific research requirements. Our flexible approach allows for customization based on your project's immunological focus. We encourage you to reach out to discuss your specific needs and the best fit for your research.
Q2: How does this service compare to generating dendritic cells from monocytes?
A2: While monocyte-derived DCs (moDCs) are commonly used, CD34⁺-derived DCs offer the advantage of generating more "natural" DC subsets (cDCs and pDCs) which often exhibit superior antigen cross-presentation and T-cell priming capabilities. This can lead to more potent and specific immune responses, particularly in complex cancer immunotherapy applications. Contact us to learn more about which source is best for your project and its unique demands.
Q3: Are there any specific precautions or considerations when using CD34⁺-derived DCs in research?
A3: As with all primary cell types, handling requires strict aseptic technique and careful adherence to optimized culture conditions to maintain optimal cell viability and function. Specific media formulations and cytokine requirements are crucial for proper differentiation and maturation. Our detailed reports provide all necessary guidelines, and our experienced scientific team is always available to offer expert support and guidance.
Q4: Can Creative Biolabs customize the differentiation protocol for unique research objectives?
A4: Absolutely. Our service is highly flexible, and we routinely customize differentiation protocols, cytokine cocktails, and maturation strategies to meet unique research objectives. Whether you require specific phenotypic markers, enhanced functional attributes, or integration into a larger experimental design, we can work with you to design a tailored solution that precisely fits your needs.
Creative Biolabs offers a suite of complementary services to support your advanced cell therapy and immunotherapy projects:
Creative Biolabs is your trusted partner for cutting-edge bone marrow CD34⁺ derived DC: next-gen DC generation service. Our expertise ensures the delivery of high-quality, functionally potent dendritic cells, empowering your immunotherapy research and drug discovery efforts with precision and efficiency. Ready to advance your immunotherapy project with next-gen dendritic cells? Please feel free to get in touch with us. Our expert team is here to assist you with detailed information, tailored solutions, and to discuss how our services can best meet your specific research and development goals.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION