To address the significant challenges in developing effective immunotherapies and the critical need for high-quality antigen-presenting cells, Creative Biolabs provides a cost-effective monocyte derived dendritic cell (DC) production service. This service helps you accelerate immunotherapy discovery and obtain potent, high-quality antigen-presenting cells through advanced ex vivo differentiation protocols and robust quality control, streamlining your cellular therapy research.
The field of immunotherapy relies heavily on the ability of dendritic cells (DCs) to present antigens and initiate robust T cell responses. Monocyte-derived dendritic cells (Mo-DCs) are particularly valuable due to their accessibility and ability to be generated in large numbers ex vivo. Research, as highlighted in numerous studies including those on DC-based vaccines for prostate cancer and glioblastoma, consistently underscores the crucial role of potent DCs in cancer immunotherapy. Developing reliable Mo-DC production services is essential to overcome current limitations in primary DC availability and to accelerate the development of effective, personalized cellular therapies for various diseases.
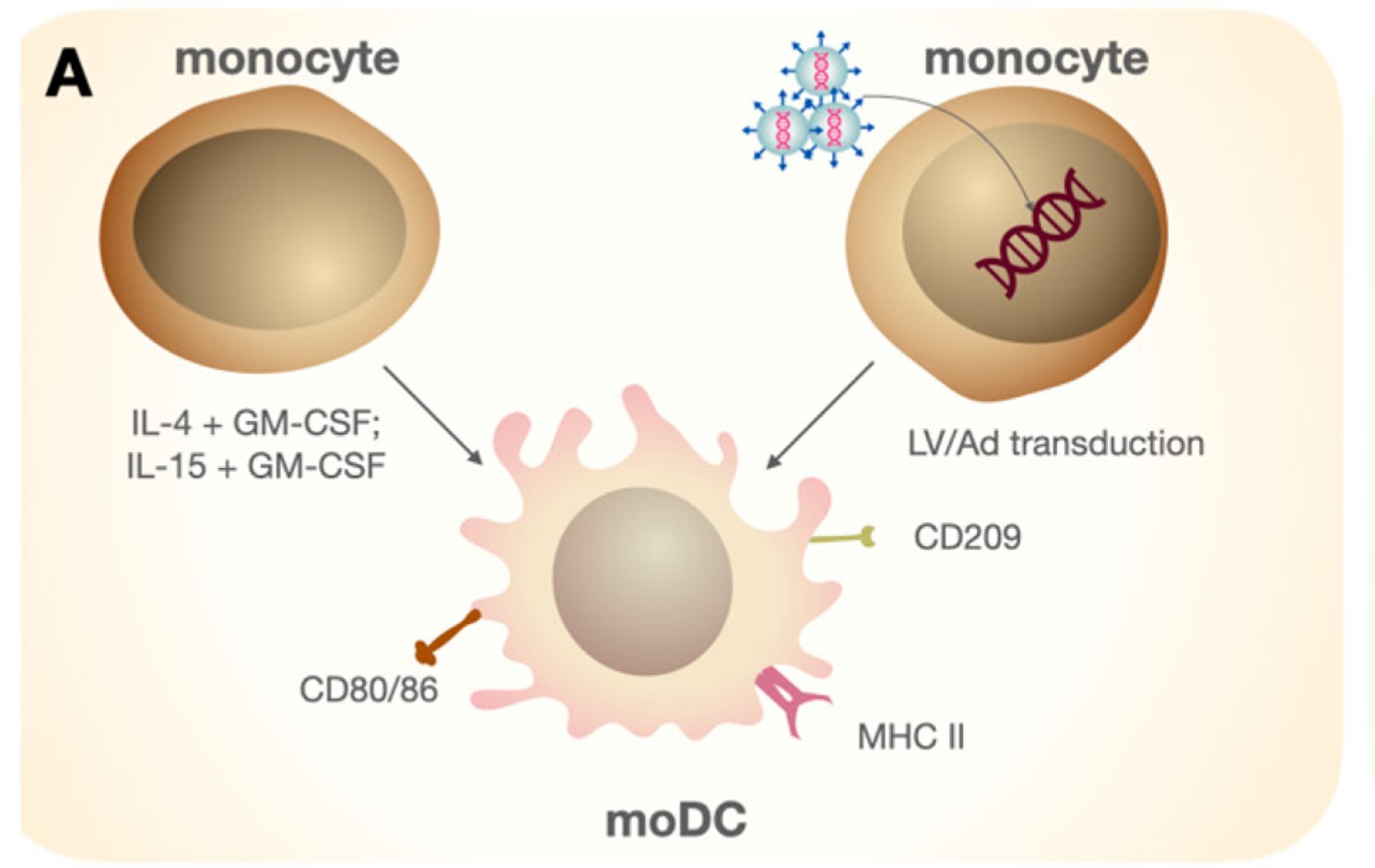 Fig.1 The direct differentiation of Mo-DC from CD14+ monocytes stimulated by various cytokines.1
Fig.1 The direct differentiation of Mo-DC from CD14+ monocytes stimulated by various cytokines.1
Creative Biolabs' monocyte derived DC production service delivers highly pure, viable, and functionally potent Mo-DCs tailored for your specific research needs. The principle behind our service involves the controlled ex vivo differentiation of patient or donor-derived PBMCs into monocytes, followed by their precise induction into immature and then mature dendritic cells using optimized cytokine cocktails and maturation stimuli. This carefully controlled process ensures the consistent generation of high-quality antigen-presenting cells.
We provide optimized solutions that simplify your cellular immunology studies, from fundamental research to preclinical development, ensuring consistent cell quality and reliable results. Our standardized protocols enable efficient execution within a typical timeframe of 3 to 5 weeks, depending on project complexity, allowing you to quickly advance your research. You can expect meticulously characterized cells with guaranteed high purity (typically >90% for key DC markers) and viability (exceeding 95% post-thaw), ready for direct application in downstream assays. This ultimately accelerates your project timelines and enhances data reproducibility, providing confidence in your experimental outcomes.
Upon receipt, fresh PBMCs are immediately processed, or cryopreserved PBMCs are carefully thawed and assessed for viability. Density gradient centrifugation or other proprietary methods are employed to ensure high-quality starting material.
We utilize advanced magnetic bead-based positive or negative selection techniques to isolate monocytes from the PBMC population. This step ensures high purity and yield of CD14+ monocytes.
Purified monocytes are cultured in specialized serum-free or low-serum media supplemented with optimal concentrations of cytokines, primarily Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) and Interleukin-4 (IL-4). Cells are typically cultured for 5-7 days under controlled conditions.
Immature DCs are stimulated with a customized cocktail of maturation-inducing agents, which may include pro-inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β) or pathogen-associated molecular patterns (PAMPs) like LPS, for an additional 24-48 hours.
Comprehensive quality control measures are performed at various stages, including cell viability assessment, cell count, purity determination by flow cytometry (e.g., CD14, CD1a, CD83, CD86, HLA-DR staining), and functional assays such as cytokine secretion profiling (e.g., IL-12, TNF-α).
Upon successful completion of all stages and quality checks, the potent Mo-DCs are cryopreserved using optimized protocols to maintain viability and functionality upon thawing.
Q1: What is the typical purity and viability of the Mo-DCs provided by Creative Biolabs?
A1: We guarantee high purity, typically greater than 90% CD11c+ and HLA-DR+ mature DCs, with viability exceeding 95% upon thawing. Our stringent quality control ensures consistent results for your downstream applications.
Q2: Can Creative Biolabs customize the DC maturation cocktail or differentiation protocol for specific research needs?
A2: Absolutely. While we have optimized standard protocols, we understand that specific research objectives may require tailored approaches. Our scientific team is adept at customizing differentiation and maturation protocols to meet your unique experimental design.
Q3: How are the functional properties of the generated Mo-DCs assessed?
A3: Beyond phenotypic markers, we assess DC functionality through various assays, including cytokine secretion profiling (e.g., IL-12), and their capacity to stimulate allogeneic T cell proliferation in mixed leukocyte reactions (MLRs). This ensures their potency as antigen-presenting cells.
Q4: What precautions should I take when handling and using the cryopreserved Mo-DCs upon receipt?
A4: We provide comprehensive guidelines for thawing and handling our cryopreserved cells to maintain optimal viability and functionality. Rapid thawing and gentle handling are key. Our support team is always available to assist with any questions.
In addition, we also produce various sourced dendritic cell production services, which include:
Creative Biolabs is your trusted partner for high-quality monocyte derived dendritic cell (DC) production, empowering your breakthroughs in cellular immunology and immunotherapy development. Our dedicated service combines scientific rigor with a client-focused approach, ensuring you receive the potent, well-characterized DCs essential for your success. If you are looking for a reliable partner to obtain high-quality Mo-DCs, please don't hesitate to get in touch with us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION