The burgeoning field of cell and gene therapies demands robust and scalable manufacturing solutions. To address the significant pain points in biopharmaceutical development, such as prolonged drug development cycles and challenges in clinical trials, Creative Biolabs provides a cost-effective large-scale dendritic cell (DC) manufacturing service: bulk production from PBMC. This service is designed to help you accelerate drug discovery and streamline clinical trial processes through advanced cell culture technologies and optimized production protocols.
Dendritic cells (DCs) are crucial for initiating and regulating immune responses, making them indispensable for advanced immunotherapies, particularly in cancer and infectious diseases. Current methods for generating DCs from peripheral blood mononuclear cells (PBMCs) often suffer from issues related to efficiency, high cost, and a lack of scalability required for clinical applications. The development of large-scale, GMP-compliant bulk DC manufacturing from PBMCs is therefore vital. This allows for the production of sufficient quantities of high-quality bulk DCs, enabling robust clinical trials and widespread therapeutic use. This ensures consistent, safe, and effective cell products for patients, accelerating the transition of DC-based immunotherapies from research to clinical reality.
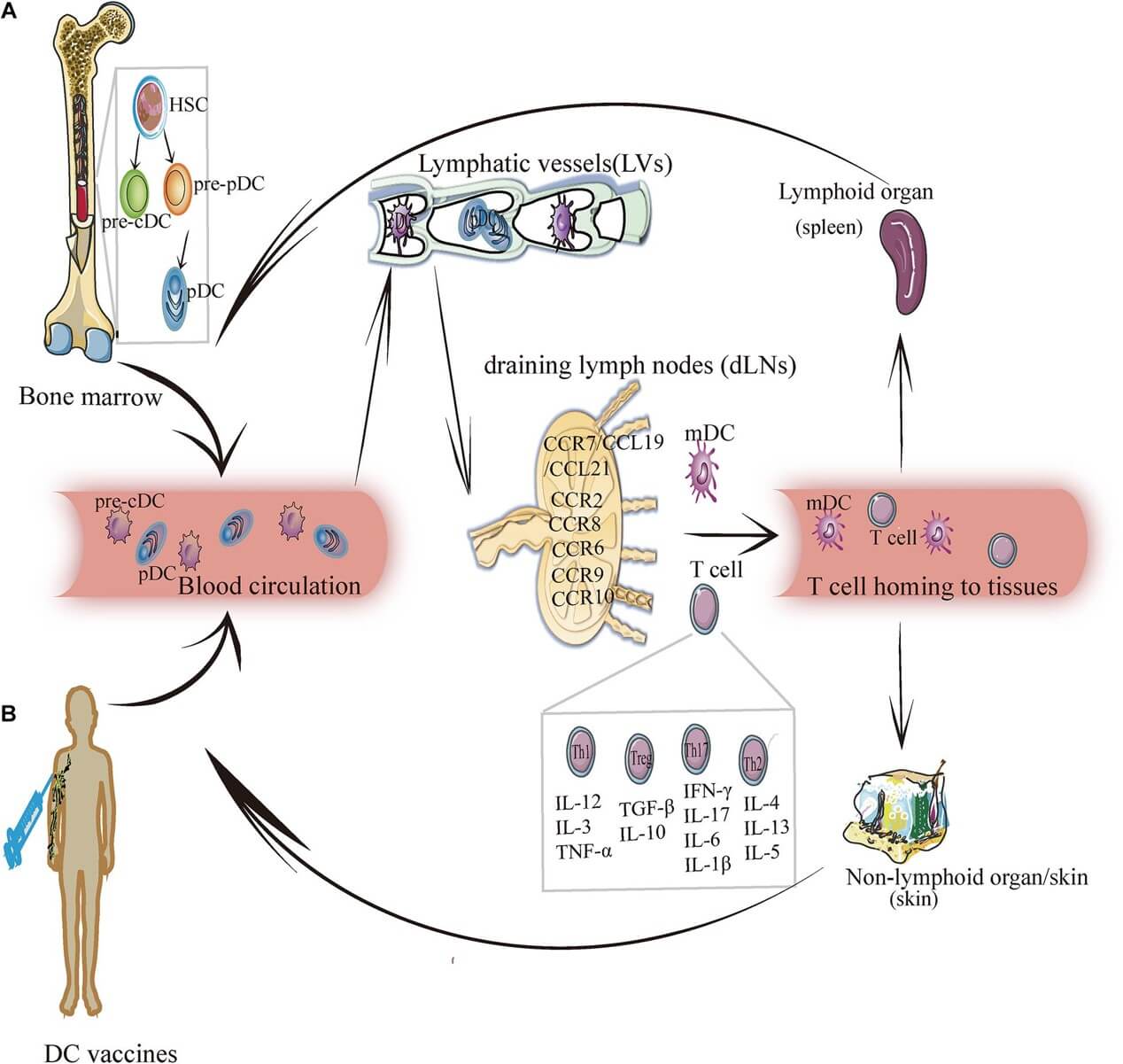 Fig.1 DC subsets' migration.1
Fig.1 DC subsets' migration.1
Creative Biolabs' large-scale dendritic cell (DC) manufacturing service offers tailored solutions to advance your immunotherapy projects. Our approach centers on the controlled differentiation and maturation of monocytes derived from peripheral blood mononuclear cells (PBMCs). Leveraging advanced cell culture techniques and optimized protocols, we consistently deliver high-purity, functionally potent dendritic cells, which are essential for robust immune responses in therapeutic applications. This service is meticulously designed to mitigate common challenges associated with in-house cell production, providing you with reliable, scalable, and quality-assured manufacturing capabilities within a typical timeframe of 4 to 6 weeks. Our commitment ensures you receive highly characterized DCs, validated for viability, purity, and functional integrity, ready to accelerate your research and clinical development.
Peripheral blood mononuclear cells (PBMCs), which contain DC precursors, are collected from a patient, typically through a process called leukapheresis.
These precursors, most commonly monocytes (CD14+ cells), are then cultured in a laboratory setting with specific cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). This process takes several days and guides the monocytes to differentiate into immature dendritic cells.
The immature DCs are then stimulated with a "maturation cocktail" of inflammatory cytokines and other stimuli. This step transforms them into mature, antigen-presenting cells that can effectively activate T-cells.
For use in cancer vaccines, the mature DCs are loaded with tumor antigens. These antigens can be in the form of tumor cell lysates, specific peptides, or mRNA encoding tumor proteins.
Throughout the entire manufacturing process, rigorous quality control checks are performed. These include comprehensive assessments of cell viability, purity (verified by extensive phenotypic analysis using multiparametric flow cytometry), stringent sterility testing, and endotoxin level quantification. Final product release is strictly contingent upon meeting our stringent, pre-defined specifications, ensuring safety and efficacy.
Q1: How does Creative Biolabs ensure the quality and consistency of its large-scale DC production?
A1: We implement rigorous quality control measures at every stage, from raw material sourcing to final product release. This includes comprehensive phenotypic analysis via flow cytometry, viability assessments, sterility testing, and endotoxin detection, all performed in a controlled, GMP-compliant environment to ensure consistent quality batch after batch.
Q2: Can Creative Biolabs customize the DC differentiation and maturation protocols for specific project requirements?
A2: Absolutely. Our service is highly flexible. We can customize cytokine cocktails, maturation agents, and culture conditions to meet your unique research or clinical specifications, whether you require immature or specifically polarized mature dendritic cells. Please reach out to discuss your specific customization needs.
To further support your research and development in cell and gene therapy, Creative Biolabs offers a suite of complementary services:
Creative Biolabs is dedicated to empowering your scientific advancements with our industry-leading large-scale DC manufacturing service. Our commitment to quality, efficiency, and customized solutions ensures that you receive the highest-grade dendritic cells to drive your innovative research and clinical trials forward. If you are interested in our bulk DC production service, we welcome global customers to reach out to us with your meaningful projects.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION