Imagine reprogramming your body's own immune cells to become precision-guided missiles against cancer. This is exactly what chimeric antigen receptor T-cell therapy accomplishes, representing one of the most exciting breakthroughs in modern cancer treatment. While traditional therapies like chemotherapy act like carpet bombing, potentially damaging both healthy and cancerous cells, CAR-T cells work more like smart missiles, specifically seeking out and destroying cancer cells. This revolutionary approach has already saved thousands of lives and continues to evolve, pushing the boundaries of what's possible in cancer treatment.
To understand CAR-T cell therapy, think of it as giving your immune system's T cells a new pair of eyes and enhanced weapons. In our immune system, T cells naturally fight infections and diseases, including cancer. However, they sometimes struggle to recognize cancer cells, which often disguise themselves to avoid detection. Cancer cells are masters of disguise, often changing their surface proteins or hiding their identifying markers, making them invisible to normal T cells. This is where the engineering of CAR-T cells comes into play.
Chimeric antigen receptor (CAR) T-cell therapy involves collecting a patient's T cells and modifying them in a laboratory to express special receptors on their surface. These receptors, called chimeric antigen receptors, work like sophisticated targeting systems. The term "chimeric" comes from Greek mythology's Chimera, a creature composed of parts from different animals. Similarly, these receptors are hybrid molecules, combining different parts of immune system proteins - imagine combining the best features of different tools to create a superior instrument.
The CAR molecule itself is an engineering marvel, consisting of three key parts working in perfect harmony. The external sensor, known as the single-chain variable fragment (scFv), acts like a highly specialized antenna that can recognize specific markers on cancer cells. This sensor is typically designed to target specific proteins found abundantly on cancer cells but rarely on healthy cells. For example, in B-cell lymphomas, the target is often CD19, a protein found almost exclusively on B cells.
The bridging structure, or transmembrane domain, does more than just connect the external and internal parts. It's carefully engineered to ensure the receptor maintains the right shape and flexibility, much like the suspension system of a high-performance vehicle. This structural stability is crucial for the receptor to function properly.
The internal activation system is perhaps the most sophisticated part, containing multiple signaling domains that work together like a complex circuit board. When the external sensor detects its target, these internal components trigger a cascade of signals within the T cell, transforming it from a passive observer into an active killer of cancer cells.
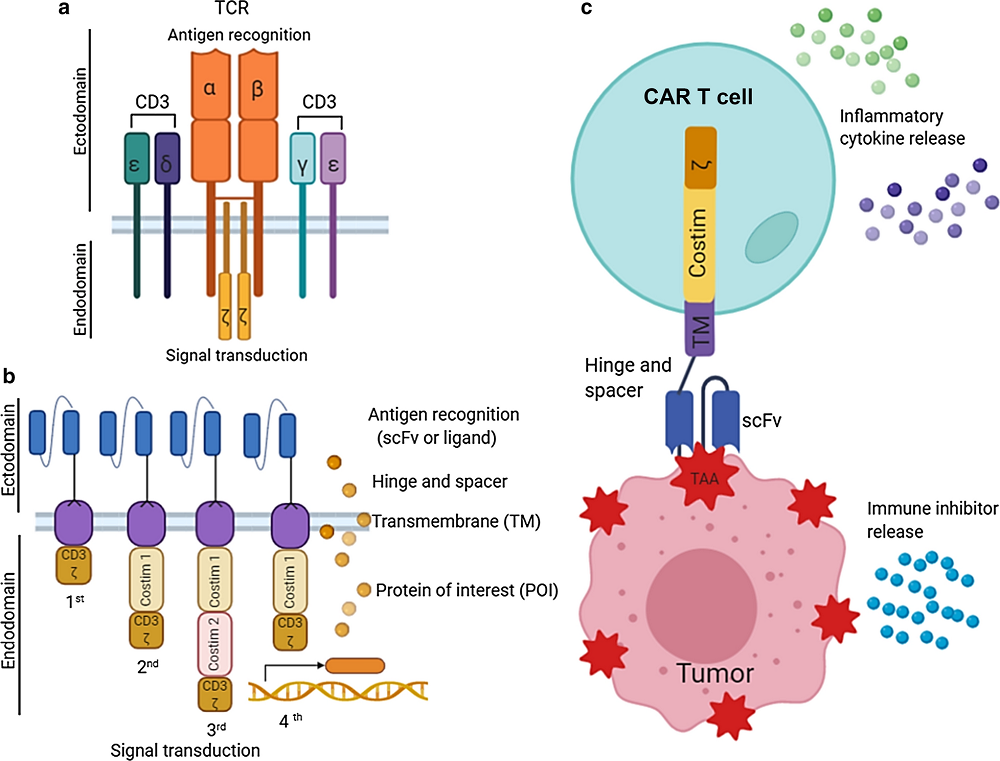 Fig.1 Basic principle of CAR structure and CAR T-cell therapy. a T-cell receptor (TCR) complex. b Basic 1st–4th generation design of CAR. c Mechanism of CAR T-cell therapy1,4.
Fig.1 Basic principle of CAR structure and CAR T-cell therapy. a T-cell receptor (TCR) complex. b Basic 1st–4th generation design of CAR. c Mechanism of CAR T-cell therapy1,4.
Over time, scientists have refined this design through several generations, each more sophisticated than the last. First-generation CARs were like early mobile phones - they could do the basic job but had limited functionality. Modern CARs are more like smartphones, incorporating multiple features that enhance their performance. Second-generation CARs added co-stimulatory domains that help the T cells persist longer and function better. Third-generation CARs went even further, including multiple stimulatory signals to enhance cell activation and survival. The latest fourth-generation CARs, sometimes called TRUCK (T cells Redirected for Universal Cytokine-mediated Killing) cells, can even produce additional immune-stimulating molecules, acting not just as soldiers but as field commanders that can coordinate a broader immune response against cancer. The newest fifth-generation CARs incorporate additional signaling domains, particularly the IL-2Rβ chain, which can directly activate the STAT3 signaling pathway to achieve superior anti-tumor responses while maintaining better control over cell activity.
The CAR T cell mechanism of action is a sophisticated process that combines precision medicine with cellular engineering. Let's break down this complex journey from regular T cells to cancer-fighting super soldiers.
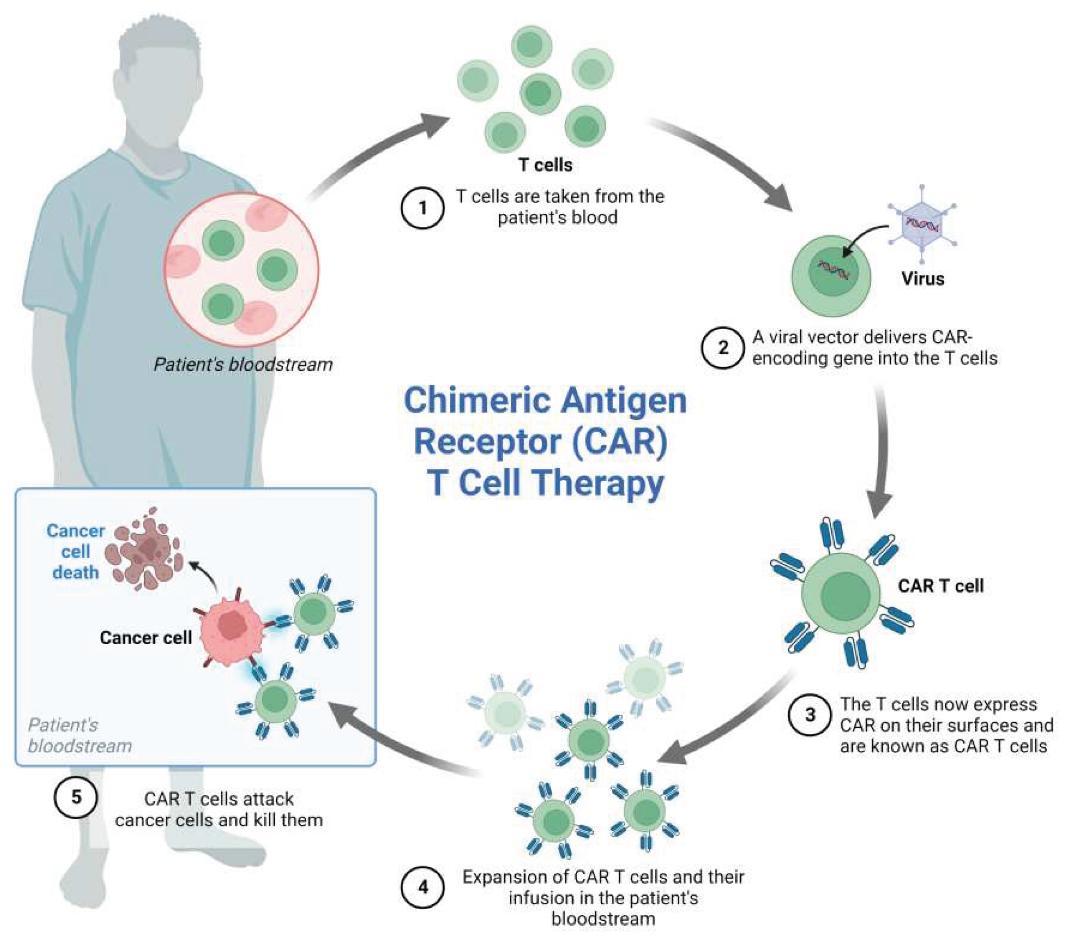 Fig.2 CAR-T cell generation and mechanism of action2,4.
Fig.2 CAR-T cell generation and mechanism of action2,4.
The journey begins with apheresis, a specialized blood collection process that can take several hours. During this procedure, the patient's blood is carefully processed through a machine that separates different blood components. Think of it as a highly sophisticated sorting system, like a gold panning operation that separates valuable nuggets (T cells) from the rest of the river material (other blood components).
The timing of this collection is crucial. Doctors must carefully consider the patient's recent treatments and overall health status to ensure they collect enough healthy T cells. Sometimes, patients need to stop certain medications temporarily to improve the quality of collected cells. The collected T cells are then cryopreserved and shipped to specialized manufacturing facilities, maintained at precise temperatures throughout their journey.
In the laboratory, the transformation of regular T cells into CAR-T cells involves multiple sophisticated steps. First, the T cells are activated using specialized beads coated with antibodies that mimic the body's natural T cell activation signals. This activation is like waking up sleeping soldiers and preparing them for training.
The genetic modification process uses viral vectors, typically modified HIV or lentivirus, stripped of their harmful components and repurposed to deliver the CAR genes. These viral vectors are like molecular delivery trucks, specially designed to insert the new genetic material without damaging the cell. The process must be precisely controlled - too little modification means ineffective CAR-T cells, while too much can stress the cells and reduce their functionality.
During the manufacturing process, the cells are grown in special bioreactors that provide optimal conditions for expansion. These systems carefully control temperature, pH, oxygen levels, and nutrients, allowing the cells to multiply while maintaining their cancer-fighting capabilities. The manufacturing process typically takes 2-3 weeks, during which the cells are constantly monitored for quality and quantity.
While the CAR-T cells are being manufactured, patients typically undergo lymphodepletion chemotherapy. This process is like preparing the soil before planting a garden - it creates space and optimal conditions for the CAR-T cells to grow and function. The timing and intensity of this preparatory treatment are crucial; too much can leave the patient vulnerable to infections, while too little might result in poor CAR-T cell expansion.
When the engineered cells are ready, they're carefully transported back to the treatment center in temperature-controlled containers. Before infusion, each batch undergoes rigorous quality testing to ensure safety and potency. The actual infusion process is relatively simple, similar to a blood transfusion, but the events that follow are remarkably complex.
Once in the bloodstream, the CAR T cells begin their search-and-destroy mission. When they encounter their target cancer cells, multiple events occur simultaneously:
The CAR receptor recognizes its target and activates the T cell, triggering the release of cytotoxic granules containing perforin and granzymes. These molecules work together like a specialized weapons system - perforin creates holes in the cancer cell membrane, while granzymes enter through these holes and trigger cell death.
The activated CAR-T cells also release signaling molecules called cytokines, which help coordinate the immune response and recruit other immune cells to join the fight. This creates a multiplier effect, where the initial response becomes amplified and more effective over time.
Perhaps most remarkably, when CAR-T cells successfully engage with cancer cells, they undergo rapid proliferation, creating an expanding army of cancer-fighting cells. This expansion phase is crucial for the therapy's success and can result in a single CAR-T cell giving rise to thousands of daughter cells, all programmed to fight cancer.
The field of CAR-T cell immunotherapy has evolved to encompass two main approaches, autologous and allogeneic, each with its own unique characteristics and applications. Understanding these different approaches is crucial for healthcare professionals and researchers working in this rapidly evolving field.
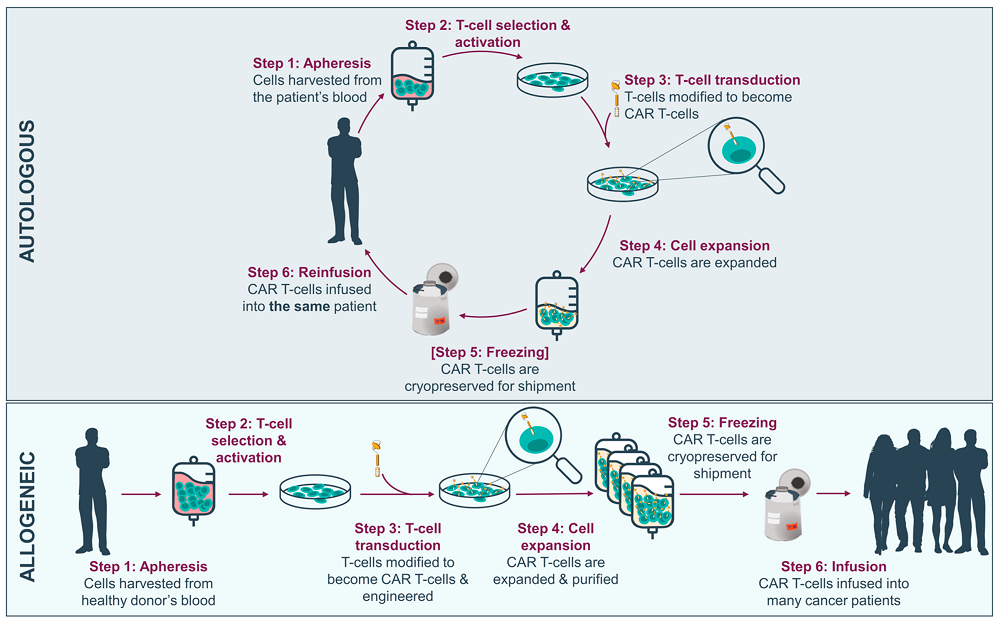 Fig.3 Overview of autologous versus allogeneic CAR-T manufacturing process from peripheral blood mononuclear cells3,4.
Fig.3 Overview of autologous versus allogeneic CAR-T manufacturing process from peripheral blood mononuclear cells3,4.
Autologous CAR T cell therapy represents the current standard of care in the field. Think of it as creating a bespoke suit - every aspect is tailored to the individual patient. The process begins with collecting T cells from the patient's own blood, a bit like taking precise measurements for that custom suit. These cells already know how to work within the patient's immune system, making them ideal candidates for modification.
The advantages of this personalized approach are significant. Since the cells come from the patient's own body, they're unlikely to be rejected by the immune system. This compatibility means the cells can potentially persist in the body for months or even years, providing ongoing surveillance against cancer recurrence. It's like having a permanent security force that knows exactly what to look for and how to respond.
However, the autologous approach faces several challenges that impact its widespread implementation. The manufacturing process is complex and time-consuming, typically taking 2-3 weeks from collection to reinfusion. During this time, patients must be carefully monitored, and their disease managed appropriately. The process is also highly dependent on the quality of the patient's T cells. If a patient has undergone multiple rounds of chemotherapy or has an aggressive disease, their T cells might be too depleted or damaged to use effectively.
The cost of autologous CAR-T cell therapy is another significant consideration. The personalized nature of the treatment, combined with the sophisticated manufacturing process, results in prices that can exceed $400,000 per treatment. This high cost poses challenges for healthcare systems and insurance providers, potentially limiting access to this revolutionary therapy.
Allogeneic CAR T therapy represents an exciting alternative approach that could potentially transform the accessibility of CAR-T cell therapy. Instead of using the patient's own cells, this approach uses T cells from healthy donors, modified and manufactured in advance. Think of it as having a ready-to-wear collection of cancer-fighting cells, available whenever needed.
The manufacturing process for allogeneic CAR-T cells involves selecting healthy donors with optimal T cell characteristics. These cells are then modified not only to express the CAR but also to minimize the risk of graft-versus-host disease (GvHD). This additional engineering often includes removing or modifying the native T cell receptor and other molecules that could cause adverse reactions in the recipient.
One of the most significant advantages of the allogeneic approach is its potential to reduce both the time and cost of treatment. By manufacturing cells in larger batches and having them ready for immediate use, the therapy could become more accessible to patients who need it urgently. This could be particularly important for patients with rapidly progressing disease who can't wait for autologous cell manufacturing.
However, allogeneic CAR-T cells face their own set of challenges. The most significant is the risk of rejection by the patient's immune system or the development of GvHD. Scientists are working on various strategies to address these challenges, including:
The use of gene editing technologies to remove proteins that could trigger immune responses or cause GvHD. This is like giving the cells a diplomatic passport that allows them to operate freely in any patient's body.
The development of safety switches that can be activated to eliminate the CAR-T cells if adverse reactions occur. Think of it as installing an emergency brake system that can be pulled if needed.
The incorporation of mechanisms to protect the CAR-T cells from the patient's immune system while maintaining their cancer-fighting abilities. This is similar to giving the cells protective armor that allows them to carry out their mission without being destroyed by the host's defenses.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION