Cell and Gene Therapies (CGT) represent a fundamental disruption to the established paradigms of medical treatment. Moving beyond the chronic management of symptoms that defines much of traditional pharmacology, these modalities aim to address the underlying etiology of disease at the molecular and cellular levels. This therapeutic class, which involves the administration of genetically modified cells or the direct correction of genetic material, offers the potential for durable, single-treatment cures for a range of conditions, including hematologic malignancies, monogenic disorders, and degenerative diseases, that were previously considered intractable. As a result, CGT has firmly emerged as the third major pillar of therapeutic technology, following small molecules and biologics, with the FDA forecasting that 10-20 new CGT products will be approved annually by 2025.
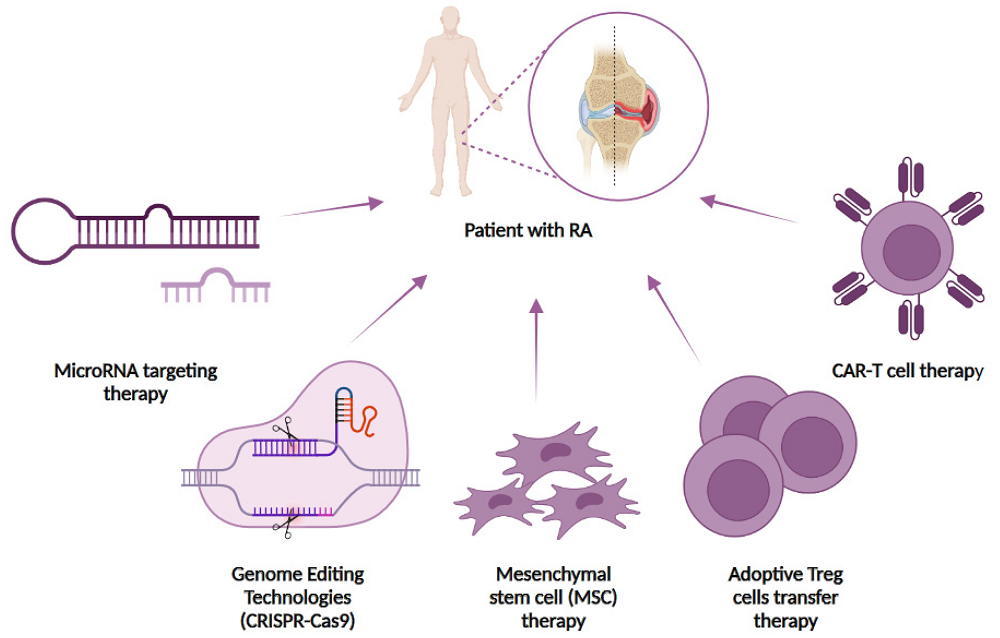 Fig.1 Cell-based and genome-editing therapies for the treatment of rheumatoid arthritis (RA)1,2
Fig.1 Cell-based and genome-editing therapies for the treatment of rheumatoid arthritis (RA)1,2
The clinical and commercial landscape is expanding at an unprecedented rate, driven by continuous innovation in gene editing tools like CRISPR-Cas9, advanced viral vector delivery systems, and sophisticated immune cell engineering. However, the profound therapeutic promise of CGT is matched by unique and formidable challenges. The development and delivery of these "living drugs" involve complex, highly individualized manufacturing processes, stringent regulatory oversight, and complex logistical supply chains. Furthermore, their high upfront costs, often ranging from hundreds of thousands to millions of dollars per treatment, create significant hurdles for patient access and healthcare system reimbursement.
This resource provides a detailed, multi-faceted analysis of the CGT field. The following sections are organized to guide readers from the core scientific principles and landmark clinical results that define the field, through the critical challenges of manufacturing and commercialization, and into a focused examination of its application in a major chronic disease.
This section analyzes the fundamental science, key technological pillars, and landmark clinical results that form the foundation of the CGT field. It provides a comprehensive technical understanding of the mechanisms of action, the spectrum of diseases being targeted, and the significant therapeutic progress achieved to date. The content moves from defining core concepts—such as the distinction between autologous and allogeneic cell therapies or in-vivo versus ex-vivo gene editing—to exploring the real-world impact of approved therapies.
The articles herein address critical questions regarding the core technologies, such as the evolution and application of Chimeric Antigen Receptor (CAR) T-cell therapy and the revolutionary potential of CRISPR-based genome editing tools. This collection also examines the expanding scope of CGT into new therapeutic areas, including central nervous system disorders, autoimmune diseases, and cardiovascular conditions. Finally, it provides a sober assessment of the associated risks, including immunogenicity and off-target effects, and the ethical frameworks required to govern their use.
This collection of articles examines the unique and formidable challenges in translating cell and gene therapies from laboratory protocols to scalable, commercially viable products. The production of these therapies necessitates a radical departure from traditional pharmaceutical manufacturing due to the inherent biological variability of the cellular starting material and the requirement for aseptic processing throughout the entire workflow. This analysis covers the entire "vein-to-vein" journey, detailing the critical role of a robust Chemistry, Manufacturing, and Controls (CMC) strategy and adherence to Good Manufacturing Practice (GMP).
The articles explore the complex technical processes of cell isolation, genetic modification, expansion, and cryopreservation, as well as the specialized infrastructure required, such as ISO 5 cleanrooms for key operations. They also analyze the business ecosystem, including the vital function of Contract Development and Manufacturing Organizations (CDMOs) in providing the expertise and capacity needed to scale production. Finally, this section confronts the complex economic realities of the field, dissecting the high cost of production and detailing the innovative, outcomes-based reimbursement models being implemented in the U.S. and Europe to manage costs and ensure patient access.
This section provides a focused case study on the application of cell therapy to diabetes mellitus, a chronic disease affecting over 537 million people globally and a leading cause of complications such as kidney failure and blindness. Traditional diabetes care is centered on lifelong disease management, which treats symptoms but does not address the root cause: the loss or dysfunction of insulin-producing pancreatic β-cells. Cell-based interventions offer a potentially curative strategy by aiming to restore this critical cell population, thereby re-establishing the body's natural glucose regulation system.
The following articles present a complete analysis of this therapeutic approach. They examine the underlying mechanisms of action for different strategies, including replacing lost cells with stem cell-derived islets and protecting remaining cells through immunomodulation. The collection traces the R&D pathway from foundational laboratory research to ongoing clinical trials, and provides a global map of development efforts in North America, Europe, and Asia. This deep dive serves as a powerful example of how CGT is being tailored to address the specific pathology of a complex, widespread disease.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION