Dysregulation in lipid metabolism is among the most prominent metabolic alterations in cancer. Lipids could serve as mediators of cancer-relevant phenotypes that promote transformation and tumor growth, invasion, and metastasis. Not only cancer cells but also the entire population of immune and stromal cells in the tumor microenvironment (TME) could impact cancer progression. Fatty acids secreted by tumor-associated stromal cells can have a tumor-promoting effect on many of the immune cells that are recruited to the TME, including macrophages, natural killer cells, dendritic cells (DCs), neutrophils, and T cells. Moreover, interactions between cancer cells and adjacent immune cells through altered lipid metabolism are known to support tumor growth and progression.
Lipid metabolism presents as a network of pathways with flexibility, feedback loops, and crosstalk that tunes to meet the increased metabolic requirements in cancer cells. Lipid metabolic reprogramming includes lipid uptake, de novo fatty acid synthesis (FAS), and fatty acid oxidation (FAO). Uncontrolled proliferation of cancer cells necessitates the accumulation of a significant quantity of lipids to make up the membranes and organelles of these cells - these lipids can be acquired from exogenous sources or synthesized endogenously through lipogenic pathways. The principal source of fatty acids in cancer cells derives largely from de novo synthesis rather than the TME. Many cancer cells have been found to upregulate enzymes involved in lipid and cholesterol biosynthesis. Tumors frequently present the capability to activate the de novo synthesis of cholesterol and fatty acids making them more independent from externally provided lipids.
FAO is an important bioenergetic pathway in many cancers that promotes proliferation, metastasis, stemness, and resistance to treatment. Tumor-associated macrophages (TAMs) tend to express elevated levels of the scavenger receptor CD36, accumulate lipids, and use FAO instead of glycolysis for energy. They also upregulate the FAS to generate more reactive oxygen species (ROS) and higher levels of cytokines which ultimately promote tumor cell survival, metastasis, angiogenesis, and immune suppression. DCs in the tumor milieu increase their cellular lipids by absorbing lipids from the environment and by increasing fatty acids synthesis. An enrichment of cellular lipid is related to a compromised capacity of DCs to assist in the activation of TIL. Cancer-associated fibroblasts (CAFs) can secret fatty acids that are taken up by tumor cells for the synthesis of other lipids.
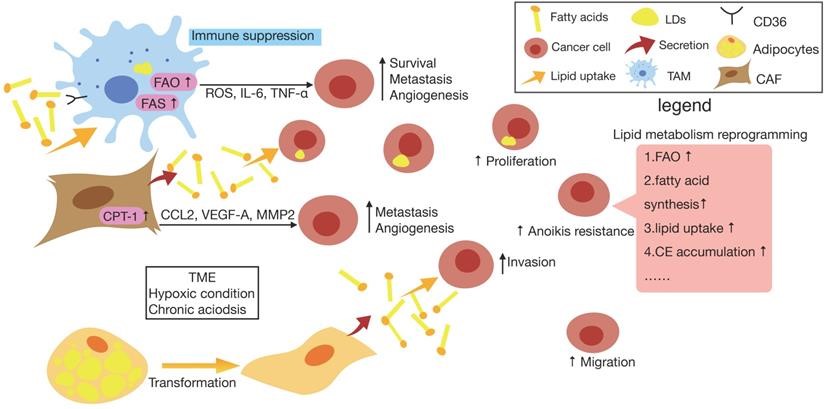 Fig.1 Lipid metabolism reprogramming of cancer cells and surrounding cells in TME. (Yu, 2021)
Fig.1 Lipid metabolism reprogramming of cancer cells and surrounding cells in TME. (Yu, 2021)
Given the extensive role of FAs in cancer pathogenesis, there is substantial clinical interest in developing therapies that target FA metabolic reprogramming. In recent years, the inhibitors targeting major participants in lipid metabolisms such as fatty acids synthesis, FAO, and cholesterol metabolism have been broadly tested in cancer treatment. The way to block fatty acid synthesis by directly targeting the enzymes including fatty acid synthase (FASN), ATP-citrate lyase (ACLY) and acetyl-CoA carboxylase (ACC) has been shown to inhibit cancer cell growth and proliferation. Because most lipid chaperones are membrane receptor proteins, targeting fatty acid uptake pathways also represents a promising strategy for cancer therapy. Exogenous fatty acid uptake largely depends on CD36 expression, the application of CD36 inhibitors in preclinical studies impedes the growth of multiple cancers. In addition, tumor immunotherapy has emerged as a revolutionary therapeutic approach, aiming at improving anti-tumor immune responses with fewer off-target effects. Further analysis of cancer lipid metabolism using more advanced technology is also required to develop novel cancer treatments.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION