Acidic condition is one of the most significant characteristics of the tumor microenvironment (TME). The Warburg effect increases the conversion of glucose to lactate in relation to low oxidative profile due to aerobic glycolysis in tumor cells. High lactate concentrations are produced by tumor cells and excreted by monocarboxylate transporters (MCT) from tumor cells at high rates, shaping acidic TME. As a matter of fact, high lactate production remodels the TME by contributing to acidosis, acting as a cancer cell metabolic fuel and inducing immunosuppression resulting in aggressive proliferation, invasion, migration and resistance therapy. Because of lactate's emerging and critical role, targeting lactate production and its transporters is important for preventing and managing tumorigenesis.
Lactate can be incorporated into the tricarboxylic acid (TCA) cycle and be a source of energy. Hypoxic TME results in the metabolic symbiosis between hypoxic and normoxic compartments of the tumor. Lactate products produced by hypoxic cells can be used as a metabolic fuel by normoxic cells through oxidative phosphorylation
Apart from their direct starvation effect, lactate also inhibits immune cells function and induces immunosuppressive TME. The production of lactate in tumor cells impacts interferon-gamma production by tumor-infiltrating T cells, NK activation, and the proportion of myeloid-derived suppressor cells. High concentrations of lactic acid in the TME block lactic acid export by T cells, thereby disturbing their metabolism and function. Furthermore, lactate derived from tumors induces M2-like polarization of tumor-associated macrophages (TAMs) via induction of HIF-1α targets VEGF and arginase, further dampening antitumor immunity.
The acidic condition increases the expression of proangiogenic factors IL-8 and VEGF and stimulates angiogenesis, both important involved in cancer metastasis. Besides lactate accumulation in the primary tumor site, its immunosuppressive properties can outspread to distant sites, thus stimulating invasion and metastasis in a paracrine fashion.
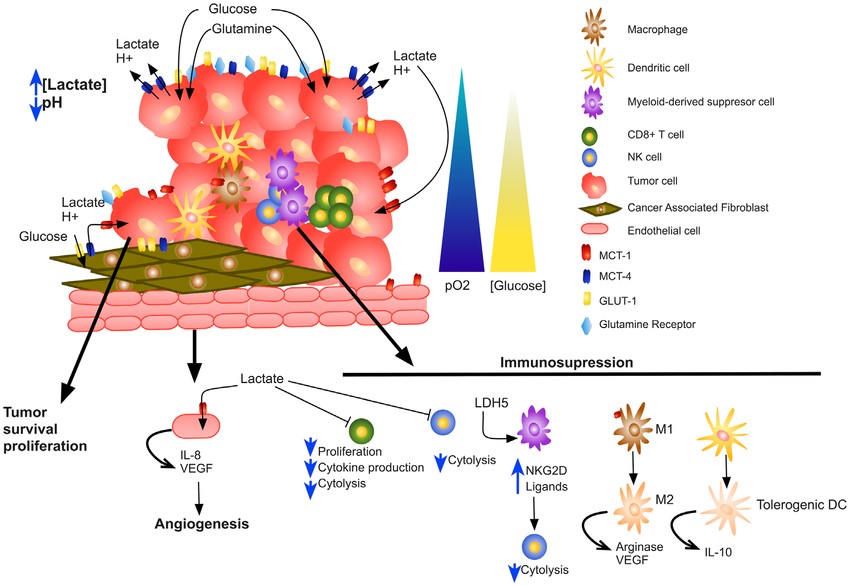 Fig.1 Impact of lactate on TME. (Romero-Garcia, 2016)
Fig.1 Impact of lactate on TME. (Romero-Garcia, 2016)
Due to the multitude of effects of lactate in promoting immune evasion of tumors and stimulating tumor angiogenesis, targeting lactate metabolism in combination with immunotherapy is a promising approach to enhance the efficacy of immune therapies. Now some drugs targeting lactate to treat tumors are being developed. Strategies that decrease lactate accumulation either through inhibiting lactate-producing enzyme lactate dehydrogenase (LDH), inhibiting the lactate transporters MCT1/4, or neutralizing lactic acid-induced acidity (oral bicarbonate supplementation) have proved effective at improving anti-tumor immune cells.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION