Limited efficacy of current immunotherapies and NK cell suppression in challenging microenvironments are common challenges. Our TGFβRII-modified NK Cell Development Service significantly enhances immune response and overcomes resistance through advanced NK cell engineering and targeted TGFβRII modification.
The rapidly evolving field of gene therapy holds immense promise for treating complex diseases, particularly cancer. Natural Killer (NK) cells are pivotal components of the innate immune system, recognized for their potent anti-tumor and anti-viral capabilities. However, their therapeutic potential is frequently hampered by immunosuppressive factors within the disease microenvironment, notably transforming growth factor-beta (TGF-β). Research demonstrates that TGF-β profoundly inhibits NK cell proliferation, cytokine production, and cytotoxicity. This inhibition reduces the efficacy and duration of NK cell-based immunotherapies. Developing TGFβRII-modified NK cells is therefore crucial to bypass this resistance mechanism, unlocking their full therapeutic potential for robust immune response improvement.
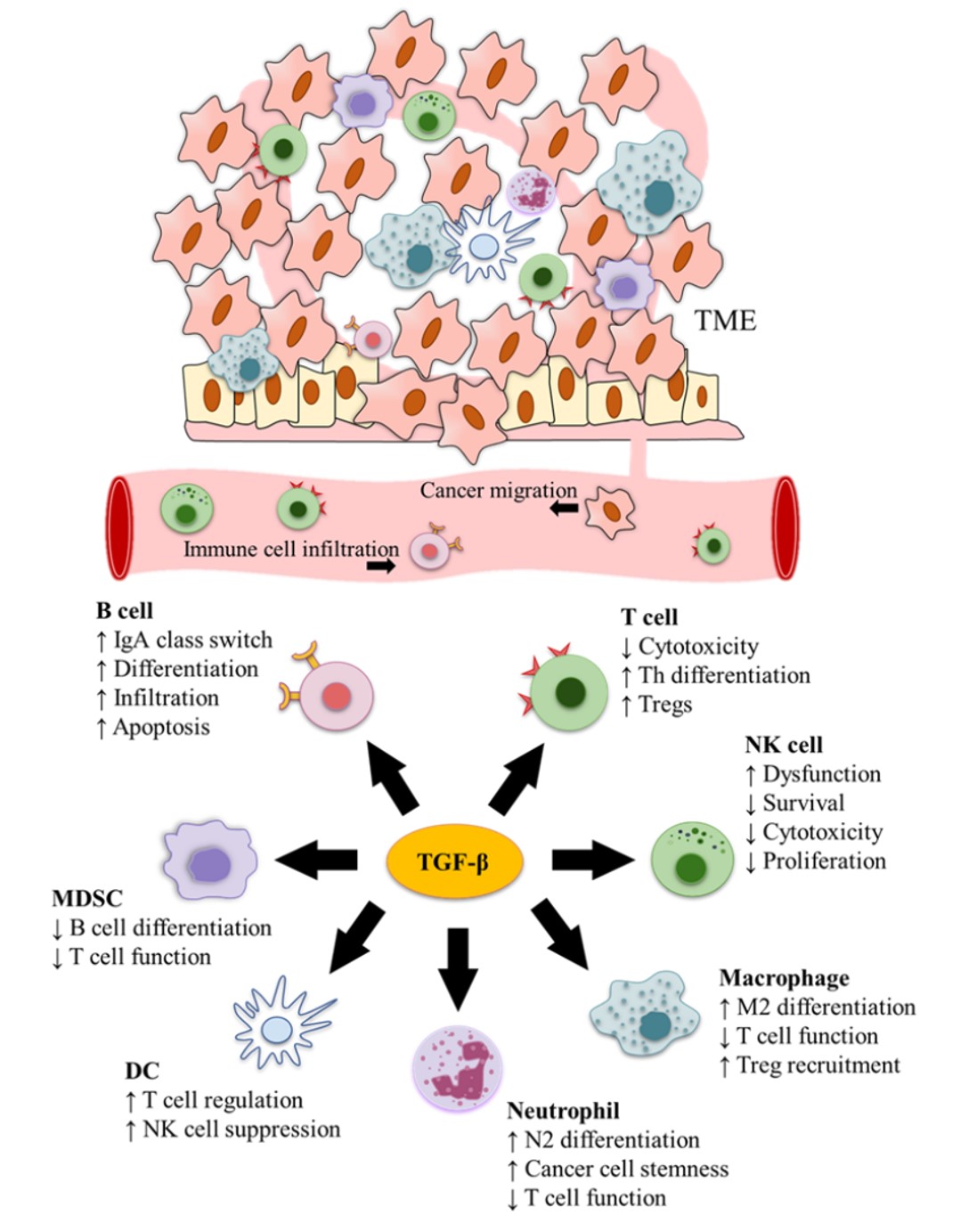 Fig.1 TGF-β plays a crucial role in cancer immune surveillance.1
Fig.1 TGF-β plays a crucial role in cancer immune surveillance.1
Creative Biolabs offers a comprehensive TGFβRII-modified NK cell development service designed to empower your immunotherapy projects with highly potent and resistant NK cell populations. We provide tailored solutions to enhance immune responses, particularly in challenging immunosuppressive environments.
Highly pure NK cell populations are isolated from the provided source material using advanced cell separation techniques. These cells are then expanded ex vivo under optimized conditions to achieve sufficient cell numbers while maintaining their viability and functionality.
Utilizing state-of-the-art gene editing technologies (e.g., lentiviral transduction), the TGFβRII gene is precisely modified within the NK cells. This modification protects NK cells from TGF-β's immunosuppressive effects.
Modified NK cells are subjected to thorough in vitro functional assessments. This includes cytotoxicity assays against target cells in the presence of TGF-β, cytokine production analysis (e.g., IFN-γ, TNF-α), proliferation assays, and phenotypic characterization (e.g., expression of activation markers, NK cell receptors).
Strict quality control procedures are followed at every stage. This entails employing flow cytometry for sterility testing, mycoplasma identification, viability evaluation, and purity analysis of the finished cell product.
Upon successful completion of all stages, the modified NK cells are carefully cryopreserved using optimized protocols to maintain their integrity and functionality for long-term storage and shipment.
Q1: What are the typical applications of the TGFβRII-modified NK cells provided by Creative Biolabs?
A1: Our clients utilize these cells for a wide range of applications, including in vitro functional studies to understand immune evasion mechanisms, in vivopreclinical models for assessing therapeutic efficacy in immunosuppressive environments, and as a component for developing next-generation cell therapies. They are ideal for researchers seeking to overcome limitations of conventional NK cell therapies.
Q2: How does Creative Biolabs ensure the quality and functionality of the modified NK cells?
A2: We employ stringent quality control measures at every stage, from initial cell isolation to final cryopreservation. This includes comprehensive functional assays (cytotoxicity, cytokine production, proliferation), immunophenotyping, and sterility testing. Our detailed data reports provide full transparency into the quality and performance of your modified NK cells.
Q3: Can TGFβRII-modified NK cells be combined with other genetic modifications, such as CAR expression?
A3: Yes, our platform offers high flexibility. We can integrate TGFβRII modification with other genetic engineering strategies, including Chimeric Antigen Receptor (CAR) expression, to create multi-functional NK cells with enhanced targeting and resistance capabilities.
To complement our TGFβRII-modified NK cell development service and further empower your research, Creative Biolabs offers a comprehensive suite of related services designed to enhance the functionality and applicability of your NK cell designs:
Creative Biolabs is committed to helping you make immunotherapy advancements more quickly. Our TGFβRII-modified NK Cell Development Service provides a powerful solution to enhance immune responses and overcome therapeutic challenges. Partner with us to leverage our scientific expertise and cutting-edge technology, driving your projects from discovery to successful application.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION