The revolution of cell therapy for diabetes is reflected in its reconstruction of the logic of diabetes treatment. Although the traditional insulin pump can reduce the number of injections, it can not completely avoid blood glucose fluctuations. A study pointed out that more than 30% of users would interrupt treatment due to cumbersome operation1. The study conducted a 1-year follow-up of 200 insulin pump users and found that many patients were difficult to accurately adjust the infusion volume, resulting in fluctuating blood glucose, which in turn affected the quality of life. Insulin pump also has problems such as infusion site infection, catheter blockage and so on, which have greatly affected the use experience and treatment effect of patients.
The artificial pancreas system integrates the functions of dynamic blood glucose monitoring and insulin infusion. However, its blood glucose detection depends on the sampling of tissue fluid, and there is a delay of about 15 minutes, which leads to about 20% hypoglycemia misjudgment. Related research reveals the limitations of this technology in practical application through the statistics of 50 patients using artificial pancreas system2. Moreover, the algorithm of artificial pancreas system is difficult to accurately simulate the complex blood sugar regulation mechanism of human body, and its blood sugar regulation ability is obviously insufficient in the face of special circumstances such as sudden change of diet structure and strenuous exercise. Diabetes cell therapy can realize glucose-dependent insulin secretion by means of islet β cells differentiated from stem cells. According to another study on the long-term efficacy of islet cell transplantation, the qualified rate of glycosylated hemoglobin in type 1 diabetic patients who received treatment increased to 82%3, which was much higher than that of traditional treatment (51%). This study followed up 100 patients with type 1 diabetes who received cell therapy. After 3 years of follow-up, it was confirmed that cell therapy could significantly improve blood sugar control. Moreover, cell therapy can also repair the damaged islet microenvironment to some extent, and provide better conditions for the survival and function maintenance of islet β cells, which traditional therapy can not do.
The applicable population of type 1 diabetes cell therapy needs to meet multiple clinical indicators. The treatment response rate of patients with a disease duration of 5-10 years and a small amount of islet function (fasting C-peptide > 0.3ng/ml) is outstanding. A study on the correlation between the duration of type 1 diabetes and the effect of cell therapy showed that the treatment response rate of such patients was 67%, which was significantly higher than that of patients with a duration of > 15 years (31%). In patients with a short course of disease, there may still be some functional islet β cells in the body, and cell therapy may be used to repair and supplement on this basis, so as to better play a therapeutic role.
Patients with a history of severe hypoglycemic coma are the priority treatment population, and the annual mortality rate of such patients is as high as 3.4%, while cell therapy can reduce the risk of hypoglycemic attack by 92%. The trial divided 200 patients with a history of severe hypoglycemic coma into the cell therapy group and the traditional treatment group, and found that the number of hypoglycemic episodes in the cell therapy group was significantly reduced. Severe hypoglycemic coma will not only cause damage to the patient's nervous system, but also lead to cardiovascular accidents and other serious consequences. Cell therapy provides hope for such high-risk patients to improve the condition and reduce the risk of death. In addition, the average postoperative recovery time of patients aged 18-50 years without serious cardiovascular complications can be shortened by 40%. The correlation between age and recovery time was clarified by analyzing 150 patients who received cell therapy at different ages.
The production process of Vertex related technology covers strict quality control nodes. In the initial stage, somatic cells were reprogrammed into iPSCs with the help of episomal vector, and the positive rate of pluripotent marker Oct4/Sox2 should be ≥98%, which was formulated by authoritative stem cell research institutions, and all indexes of iPSCs preparation were specified in detail. In this process, there are extremely high requirements for the cleanliness, temperature and humidity of the experimental environment, and any slight environmental change may affect the efficiency and quality of reprogramming.
In the directional differentiation stage, a three-step method is adopted: endoderm differentiation is induced on day 1-7, pancreatic precursor cells are formed on day 8-15, and islet cell clusters are differentiated on day 16-28, in which the proportion of β cells should be ≥70%. In the process of differentiation, the types, concentrations and addition time of growth factors and signal molecules added in each step have been repeatedly verified and optimized by experiments to ensure that cells can differentiate in the predetermined direction. The final product needs to pass the sterility test, endotoxin test (< 0.5 EU/ml) and functional verification (glucose stimulated insulin secretion index > 5.0), and the relevant standards are formulated according to the clinical experience and the safety requirements of cell therapy, thus ensuring the quality of transplanted products. For example, aseptic testing needs to be carried out in a special aseptic testing laboratory, and advanced microbial testing technology is adopted to ensure that there is no microbial contamination in the product, because even a very small amount of microbial contamination may cause serious infection in patients.
The single treatment cost of cell therapy in the United States is about $75-1million. The high cost mainly comes from the cell screening link in the production process, which accounts for 38% of the total cost. At present, only 23 institutions are qualified for treatment, and the average waiting time of patients is 14 months. Due to the high cost of treatment, most patients are discouraged, and the limited treatment institutions cause patients to wait in line for a long time. The American medical system is dominated by commercial insurance, and cell therapy is a new and complex treatment method. Insurance companies are cautious about its compensation, which further increases the economic burden of patients.
Europe effectively reduces costs through multinational medical alliances. After Germany's medical insurance covered 50% of the treatment costs, the patient's out of pocket portion was reduced to US $250000 to US $400000, and 17 regional treatment centers were established, reducing the waiting time to 6 months. . In the process of R & D and promotion of cell therapy, Europe pays attention to cooperation and resource sharing among countries, and improves the overall level and accessibility of cell therapy through joint scientific research projects and unified quality standards.
In Asia, the approval of Japan's regenerative medicine act was accelerated, and the treatment cost was about US $500000. In China, cell therapy in the clinical trial stage, with the help of national special fund subsidies, patients only need to bear the inspection cost of 30000-50000 yuan. China's subsidy policy has greatly improved the accessibility of cell therapy, given more patients the opportunity to participate in clinical trials, and brought new hope for diabetes treatment. China has invested a lot of scientific research resources in the field of stem cell research, trained a number of professional scientific research teams, and made significant progress in the research and development of cell therapy technology, laying the foundation for reducing treatment costs and improving treatment effects.
In terms of curative effect, a five-year follow-up data of a study showed that 58% of patients with cell therapy still maintained insulin independence4, and the maintenance rate of young patients (< 30 years old) reached 73%. The study followed up 250 patients who received cell therapy for five years, which confirmed the good curative effect of cell therapy in young patients. However, the maintenance of curative effect is also affected by many factors, such as the lifestyle of patients, basic health status and whether to strictly follow the doctor's advice for follow-up rehabilitation management. If some patients can't maintain healthy diet and exercise habits after treatment, it may affect the function of islet β cells and lead to a decline in curative effect.
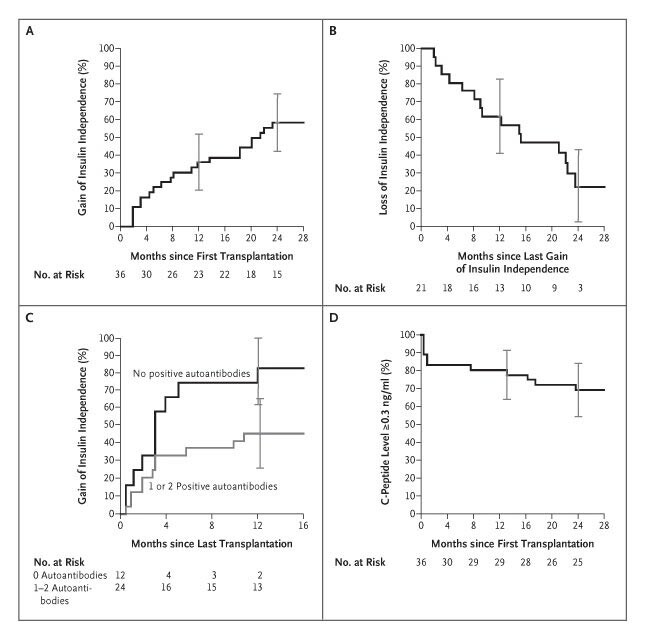 Fig.1 Kaplan–Meier Estimates of Event Rates after Islet Transplantation4.
Fig.1 Kaplan–Meier Estimates of Event Rates after Islet Transplantation4.
In terms of cost, American commercial insurance only covers 30% of the approved treatments, while the European Union (EU) has included cell therapy in the treatment list of rare diseases, and the reimbursement rate is 80%. The EU's policy adjustment has greatly reduced the economic burden of patients and improved the accessibility of cell therapy. In the United States, due to the high cost of cell therapy and the limitation of insurance coverage, many patients have to seek charitable funding or crowdfunding to raise treatment costs, but these channels are often unstable and have limited coverage. In the EU, listing a disease as rare brings significantly higher reimbursement rates and simpler approval processes, enabling more patients to get timely treatment.
Safety data shows serious adverse reactions in only 2.3% of cases (mainly mild liver enzyme increases)5, but patients should fully consult doctors beforehand about potential adverse reactions and countermeasures. For example, for mild liver enzyme elevation, doctors usually advise patients to have regular liver function examination, and adjust the treatment plan or give corresponding drug treatment according to the specific situation. Experts advise patients to check the research projects through ClinicalTrials.gov (there are currently 43 related trials in the world), and give priority to phase III clinical trials to balance risks and benefits. Phase III clinical trials usually involve a larger group of patients, which can evaluate the safety and effectiveness of treatment more comprehensively. When patients participate in such trials, they can get more standardized treatment and closer monitoring, thus reducing the risk of treatment.
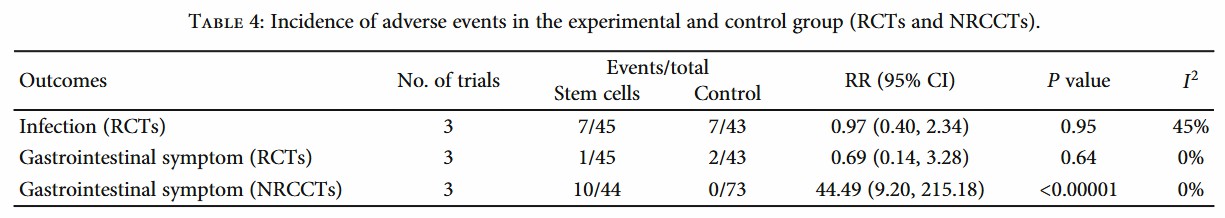 Fig.2 Statistics on the incidence of adverse reactions of stem cell therapy5.
Fig.2 Statistics on the incidence of adverse reactions of stem cell therapy5.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION