Diabetes mellitus is a global health crisis, affecting over 537 million people worldwide, with its prevalence continuing to rise. The pathology of the disease is characterized by the progressive destruction (Type 1) or dysfunction (Type 2) of insulin-producing pancreatic β-cells, leading to an inability to regulate blood glucose. Traditional care is centered on lifelong disease management through insulin therapy and other medications—approaches that control symptoms but do not halt the underlying pathology and its devastating long-term complications, such as nephropathy and retinopathy.
Cell therapy represents a fundamentally different strategy, one that aims to address the root cause of diabetes by restoring the body's deficient β-cell population. This section provides a comprehensive case study of the science, development, and application of this therapeutic approach. The articles herein detail the core scientific principle of rebuilding pancreatic islet function, trace the research and development process from foundational laboratory work to ongoing human trials, and map the current global clinical landscape. The analysis also extends to the treatment of severe diabetic complications, demonstrating the broad potential of cell-based interventions to revolutionize care for this chronic disease.
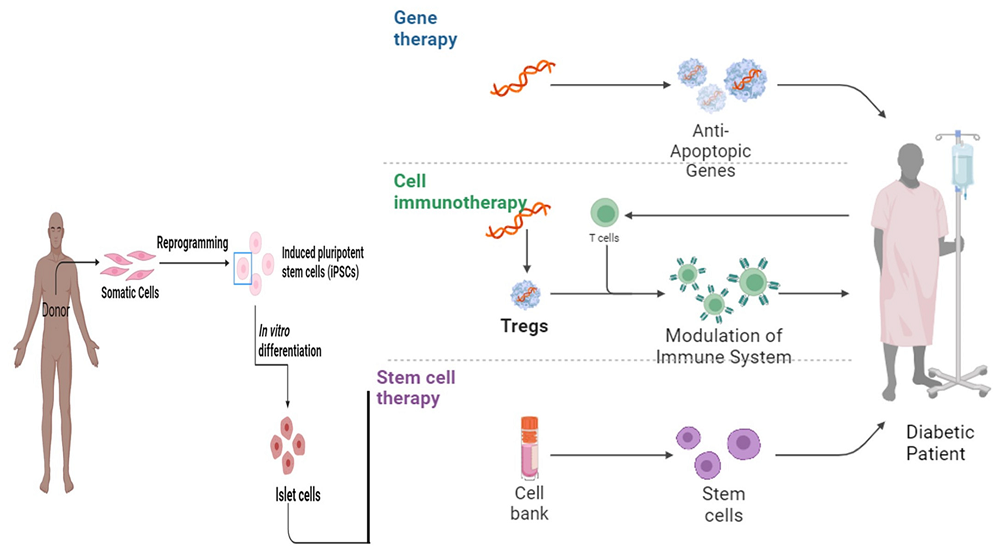 Fig.1 The role of gene therapy, cell immunotherapy, and stem cell therapy in diabetes mellitus1,2
Fig.1 The role of gene therapy, cell immunotherapy, and stem cell therapy in diabetes mellitus1,2
This article frames cell therapy as a revolutionary shift from lifelong diabetes management to a potentially curative intervention. It contrasts the limitations of traditional treatments with three core cell-based strategies: stem cell therapy to generate new β-cells from pluripotent sources, islet cell transplantation to directly replace lost cells from donors, and immune cell modulation therapy to halt the autoimmune attack in Type 1 diabetes. Furthermore, it explores the multitarget mechanisms by which stem cells can treat severe diabetic complications, detailing how they secrete factors like vascular endothelial growth factor (VEGF) to promote vascular neogenesis and remodel the immune microenvironment to heal chronic diabetic foot ulcers.
Focusing on the primary therapeutic goal of rebuilding pancreatic islet function, this piece explains how cell therapy can restore the body's natural capacity to secrete insulin. It details the two main pathways for mesenchymal stem cells (MSCs) in this context: 1) direct differentiation into insulin-producing cells (IPCs) for transplantation, and 2) a paracrine repair mechanism, where MSCs migrate to damaged islets and secrete various cytokines and growth factors to promote the regeneration of endogenous β-cells. The article also reviews encouraging results from recent clinical trials, which have shown that MSC infusion is safe and may better preserve pancreatic β-cell function in newly diagnosed Type 1 diabetes patients.
A new era of diabetes treatment: a comprehensive guide to cell therapy
This guide provides a clinical and practical analysis of cell therapy's advantages over conventional diabetes management technologies. It highlights the limitations of insulin pumps, where a study showed over 30% of users may discontinue treatment due to operational complexity, and artificial pancreas systems, which can have a 15-minute delay in glucose detection leading to potential misjudgments. The text provides a detailed look at the multi-step production and quality control process for a leading therapy, from iPSC reprogramming to final β-cell differentiation, and compares the vast disparities in global treatment costs and patient access between the United States (up to $1 million), Europe, and China (clinical trial costs of ¥30,000-50,000).
This article traces the complete research and development pathway for diabetes cell therapy, from foundational science to industrial production challenges. It reviews the evolution from early animal experiments to human trials, detailing the core technologies involved, such as the use of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) as source materials for generating new β-cells. It also explores key technical hurdles, including the challenge of improving the efficiency of directed differentiation and developing advanced transplantation techniques, such as portal vein or subcutaneous transplantation using biomaterial-based delivery systems, to ensure long-term cell survival and function.
This article presents a current snapshot of the global R&D and clinical landscape for diabetes cell therapy. It analyzes the active research in North America, where gene-editing techniques like CRISPR-Cas9 are widely used to improve transplanted cell function. It also details the unique regulatory focus of the European Medicines Agency (EMA), which emphasizes long-term data and multi-center trials. The text covers recent breakthroughs in Asia, highlighting a major advance in China where a Type 2 diabetic patient with severely impaired islet function was successfully treated and became insulin-independent for 33 months after receiving islet tissue regenerated from their own reprogrammed peripheral blood mononuclear cells.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION