Creative Biolabs is a contract research organization (CRO) solution firm established by a team of scientists with considerable expertise and experience in cell therapy research and development. We are committed to offering high-quality products and services to clients in the academic and industrial sectors across the world, supporting them in the pursuit of breakthrough scientific discoveries and further enhancing preclinical and clinical research.
CD22 is a B-cell surface molecule also known as Sialic acid-binding Ig-like lectin2 (Siglec-2). It regulates the activity and maturation of B cells mainly by interacting with sugar molecules on the cell surface. CD22 is a supramembrane glycoprotein with a full length of approximately 1350 amino acid residues. CD22 contains a short tail located on the cytoplasmic side and a large N-terminus located extracellularly. The N-terminus of CD22 is rich in Ig structural domains that can be used to bind to other proteins, including the Igα/Igβ subunit within the B-cell receptor (BCR) complex and CD22L, another Siglec family member on the natural killer (NK) cell receptor. These binding interactions can be mediated by regulating multiple key molecules in the B-cell signal transduction pathway. The intracellular tail of CD22 also contains several distinct structural domains, including a titin chain reaction sequence, a CTYC/CXXC-like zinc finger domain, and a Hormone Binding Domain (HBD). Among them, the titin chain reaction sequence and the CTYC/CXXC-like zinc finger domain function mainly to connect CD22 to the B-cell membrane and regulate its signaling pathways. Moreover, CD22 is associated with a variety of B-cell-related diseases, including acute lymphoblastic leukemia, non-Hodgkin's lymphoma, and multiple sclerosis in adults and children. Based on these findings, CD22 has emerged as a widely studied target for immunotherapy.
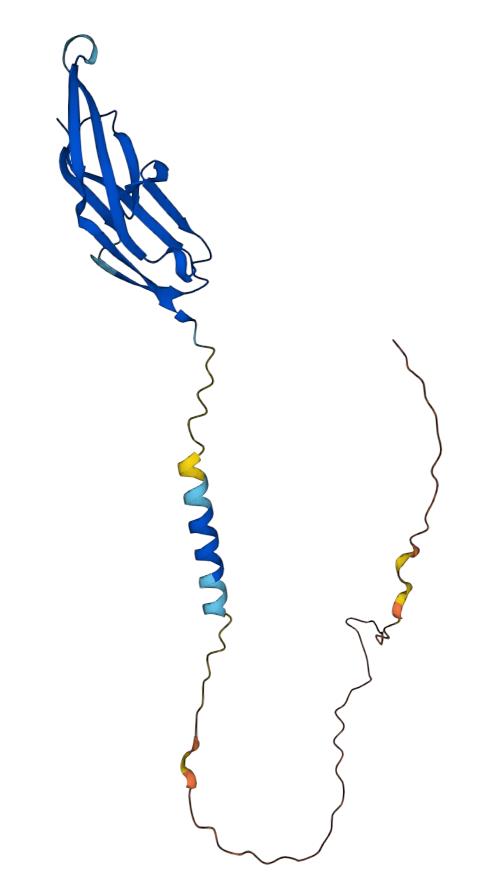 Fig.1 Structure of CD22
Fig.1 Structure of CD22
CD22 is a negative regulator that binds to Igα/Igβ subunits, thereby inhibiting BCR signaling. In the inactivated state, CD22 inhibits BCR signaling by binding directly to Igα/Igβ subunits, thereby preventing the onset of autoimmune responses. In addition, CD22 can be dissociated by phosphorylation of key enzymes in the presence of activated BCR, thereby releasing the BCR signaling pathway.
The role of CD22 played in regulating the BCR signaling pathway is an important factor affecting B cell function. Several studies have shown that CD22 is associated with autoimmune diseases and malignant lymphomas. Also, phosphorylation modifications of key CD22 enzymes are abnormal. The expression of CD22 is significantly downregulated in some autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, and autoimmune hemolytic anemia. All these abnormalities may lead to the loss of the original negative regulatory role of CD22, thereby compromising the regulatory BCR signaling pathway and leading to the development of autoimmune diseases.
The mechanism of action and expression of CD22 in B-cell lymphomas are complex. Some studies have found that the expression of CD22 is different in different types of B-cell lymphomas. CD22 also influences the development and prognosis of lymphoma by regulating the interaction of cell signaling pathways in the tumor microenvironment. The role of BCR signaling pathway in chronic lymphocytic leukemia indicates that BCR signaling pathway plays an important role in chronic lymphocytic leukemia, participating in the biological processes of leukemia cell proliferation, survival, invasion and so on. Therefore, inhibition of BCR signaling pathway is expected to be a new target for the treatment of chronic lymphocytic leukemia. In addition, studies have suggested the synergistic effect of CD22 and BCR signaling pathways in Hodgkin's lymphoma. CD22 and BCR signaling pathways have a synergistic effect in Hodgkin's lymphoma, influencing each other to affect the proliferation, survival and metastasis of tumor cells. Therefore, simultaneous targeting of CD22 and BCR signaling pathways may be one of the effective strategies for treating Hodgkin's lymphoma.
CD22 is widely used in CAR-T cell therapy as a target for the treatment of B-cell malignancies. The therapy uses modified T cells engineered to bind to specific antigen receptors to form CAR-T cells that enable them to recognize and attack excess B cells in the body. CAR-T cell therapy targeting CD22 has shown extremely high cure rates in clinical trials. CD22 There are currently a number of ongoing clinical trials in CAR-T cell therapy targeting CD22. Most of the CD22 CAR-T clinical trials are still in the early stages, including Phase I/II. CD22 CAR-T cell therapy is mainly used for the treatment of B-cell malignancies, such as acute lymphoblastic leukemia (ALL) and B-cell lymphoma. Most trials use autologous T cells or peripheral blood stem cells as the cell source. CD22 CAR-T cells can be used in combination with other target CAR-T cells to enhance the therapeutic effect. For example, in the treatment of B-cell malignancies, CD22 and CD19 are often expressed together, so the combination of CD22 and CD19 CAR-T cells can be used to improve the therapeutic effect.
Table 1. Ongoing CD22-Targeted CAR Cell Therapy Clinical Trials
| NCT Number | Title | Status | Conditions | Sponsor/Collaborators | Phases |
| NCT04715217 | Targeting CD19 and CD22 CAR-T Cells Immunotherapy in Patients With Relapsed or Refractory B Cell Lymphoma | Recruiting | Lymphoma, B-Cell | Shanxi Province Cancer Hospital|Shanghai Ultra-T Immune Therapeutics Co. LTD | Phase 1|Phase 2 |
| NCT05507827 | Safety of Myeloablative Conditioning, Orca-T, and Allogeneic, Donor-Derived CD19/CD22-CAR (Chimeric Antigen Receptor) T Cells in Adults With B-cell Acute Lymphoblastic Leukemia (ALL) | Recruiting | Lymphoid Leukemia | Crystal Mackall, MD|Orca Biosystems, Inc.|Stanford University | Phase 1 |
| NCT04088890 | Autologous CD22 CAR-T Cells in Adults w/ Recurrent or Refractory B Cell Malignancies | Recruiting | B-ALL|B-cell Non Hodgkin Lymphoma|DLBCL|Follicular Lymphoma Grade 3B | Stanford University | Phase 1 |
| NCT04788472 | Sequential CD19 and CD22 CAR-T Therapy for Newly Diagnosed Ph+ B-ALL | Recruiting | B-Cell Acute Lymphoblastic Leukemia, Adult | Zhejiang University|Yake Biotechnology Ltd. | Phase 1|Phase 2 |
| NCT04740203 | Sequential CD19 and CD22 CAR-T Therapy for Newly Diagnosed Ph- B-ALL | Recruiting | B-Cell Acute Lymphoblastic Leukemia, Adult | Zhejiang University|Yake Biotechnology Ltd. | Phase 1|Phase 2 |
| NCT04714593 | Targeting CD19 and CD22 CAR-T Cells Immunotherapy in Patients With Relapsed or Refractory Acute B Lymphocytic Leukemia | Recruiting | Acute LAymphocytic Leukemia, B-Cell | Shanxi Province Cancer Hospital|Shanghai Ultra-T Immune Therapeutics Co. LTD | Phase 1|Phase 2 |
| NCT04034446 | CD19-CD22 Chimeric Antigen Receptor T (CAR-T) Cell for Treatment of B Cell Acute Lymphoblastic Leukemia (B-ALL) | Active, not recruiting | Relapsed or Refractory B Cell Acute Lymphoblastic Leukemia | Institute of Hematology & Blood Diseases Hospital|Juventas Cell Therapy Ltd. | Early Phase 1 |
| NCT05418088 | Genetically Engineered Cells (Anti-CD19/CD20/CD22 CAR T-cells) for the Treatment of Relapsed or Refractory Lymphoid Malignancies | Recruiting | Recurrent Acute Lymphoblastic Leukemia|Recurrent B Acute Lymphoblastic Leukemia|Recurrent B-Cell Prolymphocytic Leukemia|Recurrent Chronic Lymphocytic Leukemia|Recurrent High Grade B-Cell Lymphoma|Recurrent Indolent Non-Hodgkin Lymphoma|Recurrent Non-Hodgkin Lymphoma|Recurrent Transformed Chronic Lymphocytic Leukemia|Refractory Acute Lymphoblastic Leukemia|Refractory B Acute Lymphoblastic Leukemia|Refractory B-Cell Prolymphocytic Leukemia|Refractory Chronic Lymphocytic Leukemia|Refractory High Grade B-Cell Lymphoma|Refractory Indolent Non-Hodgkin Lymphoma|Refractory Non-Hodgkin Lymphoma|Refractory Transformed Chronic Lymphocytic Leukemia | Sumithira Vasu|Ohio State University Comprehensive Cancer Center | Phase 1 |
| NCT05523661 | Dasatinib Plus Anti-CD19/CD22 Bispecific CAR-T Cell Therapy for Elderly Ph-positive ALL Patients | Recruiting | Ph Positive ALL|CAR-T Cell|Dasatinib | Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine | Phase 1 |
| NCT04499573 | Bispecific CD19/CD22 CAR-T for Treatment of Children and Young Adults With r/r B-ALL | Active, not recruiting | B-ALL | Federal Research Institute of Pediatric Hematology, Oncology and Immunology | Phase 1|Phase 2 |
| NCT04556669 | Anti-PD-L1 Armored Anti-CD22 CAR-T/CAR-TILs Targeting Patients With Solid Tumors | Recruiting | Solid Tumor, Adult|Cervical Cancer|Sarcoma|NSCLC | Hebei Senlang Biotechnology Inc., Ltd. | Phase 1 |
| NCT04029038 | Modified Immune Cells (CD19-CD22 CAR-T Cells) in Treating Patients With Recurrent or Refractory CD19 Positive, CD22 Positive Leukemia or Lymphoma | Not yet recruiting | CD19 Positive|CD22 Positive|Minimal Residual Disease|Progressive Disease|Recurrent B Acute Lymphoblastic Leukemia|Recurrent Chronic Lymphocytic Leukemia|Recurrent Non-Hodgkin Lymphoma|Refractory B Acute Lymphoblastic Leukemia|Refractory Chronic Lymphocytic Leukemia|Refractory Non-Hodgkin Lymphoma | M.D. Anderson Cancer Center|National Cancer Institute (NCI) | Phase 1|Phase 2 |
| NCT02315612 | Anti-CD22 Chimeric Receptor T Cells in Pediatric and Young Adults With Recurrent or Refractory CD22-expressing B Cell Malignancies | Recruiting | Follicular Lymphoma|ALL|NHL|Large Cell Lymphoma | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1 |
| NCT03919526 | Anti-CD19/CD22 Bispecific CAR-T Cell Therapy for MRD Positive ALL | Recruiting | MRD-positive|Acute Lymphoblastic Leukemia | Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine | Phase 1 |
| NCT03244306 | A Phase 1 Study of CD22-CAR TCell Immunotherapy for CD22+ Leukemia and Lymphoma | Active, not recruiting | Leukemia | Seattle Children's Hospital | Phase 1 |
| NCT03330691 | A Feasibility and Safety Study of Dual Specificity CD19 and CD22 CAR-T Cell Immunotherapy for CD19+CD22+ Leukemia | Recruiting | Leukemia|Lymphoma | Seattle Children's Hospital | Phase 1 |
| NCT05674175 | Co-administration of CART22-65s and huCART19 for B-ALL | Recruiting | B-cell Acute Lymphoblastic Leukemia|B Lineage Lymphoblastic Lymphoma | Stephan Grupp MD PhD|University of Pennsylvania|Children's Hospital of Philadelphia | Phase 1|Phase 2 |
| NCT04340167 | Study of Anti-CD22 CAR-T Cells Treating Leukemia Children | Recruiting | Acute Lymphoblastic Leukemia|Acute Lymphoblastic Leukemia, in Relapse|Refractory Acute Lymphoblastic Leukemia | Beijing Boren Hospital | Phase 2 |
| NCT03241940 | Phase I Dose Escalation Study of CD19/CD22 Chimeric Antigen Receptor (CAR) T Cells in Children and Young Adults With Recurrent or Refractory B Cell Malignancies | Recruiting | B Acute Lymphoblastic Leukemia|CD19 Positive|Minimal Residual Disease|Philadelphia Chromosome Positive|Recurrent Adult Acute Lymphoblastic Leukemia|Recurrent Childhood Acute Lymphoblastic Leukemia|Refractory Acute Lymphoblastic Leukemia | Crystal Mackall, MD|Stanford University | Phase 1 |
| NCT05223686 | To Evaluate the Safety and Tolerability of Human CD19-CD22 Targeted T Cells Injection for Subjects With R/R B-ALL. | Not yet recruiting | Acute Lymphoblastic Leukemia | Hrain Biotechnology Co., Ltd.|Ruijin Hospital | Phase 1 |
| NCT03233854 | CD19/CD22 Chimeric Antigen Receptor (CAR) T Cells With or Without NKTR-255 in Adults With Recurrent or Refractory B Cell Malignancies | Recruiting | B Acute Lymphoblastic Leukemia|CD19 Positive|Minimal Residual Disease|Philadelphia Chromosome Positive | Crystal Mackall, MD|California Institute for Regenerative Medicine (CIRM)|Stanford University | Phase 1 |
| NCT03448393 | CD19/CD22 Chimeric Antigen Receptor (CAR) T Cells in Children and Young Adults With Recurrent or Refractory CD19/CD22-expressing B Cell Malignancies | Recruiting | Acute Lymphoid Leukemia|B-Cell Leukemia|Leukemia, Lymphocytic, B Cell|B-Cell Lymphoma|Lymphoma, Non-Hodgkin | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1 |
| NCT05651178 | Human CD19-CD22 Targeted T Cells Injection for Refractory/Relapsed Central Nervous System Leukemia/Lymphoma Patients | Recruiting | Central Nervous System Lymphoma | Hrain Biotechnology Co., Ltd.|Second Affiliated Hospital of Nanchang University | Early Phase 1 |
| NCT05442515 | CD19/CD22 Bicistronic Chimeric Antigen Receptor (CAR) T Cells in Children and Young Adults With Recurrent or Refractory CD19/CD22-expressing B Cell Malignancies | Recruiting | Acute Lymphoid Leukemia|B-Cell Leukemia|Leukemia, Lymphocytic, B Cell|B-Cell Lymphoma|Lymphoma, Non-Hodgkin | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1|Phase 2 |
| NCT04648475 | Safety and Efficacy of CD19 and CD22 Targeted CAR-T Therapy for Relapsed/Refractory B Cell Leukemia and Lymphoma | Recruiting | Leukemia, B-cell|Lymphoma, B-Cell | Chongqing Precision Biotech Co., Ltd | Phase 1|Phase 2 |
| NCT05225831 | Safety and Efficacy of CD19/CD22 Dual Targeted CAR-T Cell Therapy in R/R B-Cell Acute Lymphoblastic Leukemia | Recruiting | CD19+ and CD 22+ B-ALL | Hebei Senlang Biotechnology Inc., Ltd.|Hebei Yanda Ludaopei Hospital | Early Phase 1 |
| NCT04714827 | Targeting CD19 and BCMA CAR-T Cells Immunotherapy in Patients With Relapsed or Refractory Multiple Myeloma | Recruiting | Multiple Myeloma | Shanxi Province Cancer Hospital|Shanghai Ultra-T Immune Therapeutics Co. LTD | Phase 1|Phase 2 |
| NCT05094206 | CAR20.19.22 T-cells in Relapsed, Refractory B-cell Malignancies | Recruiting | B-cell Non Hodgkin Lymphoma|B-cell Chronic Lymphocytic Leukemia | Medical College of Wisconsin|Miltenyi Biomedicine GmbH | Phase 1 |
| NCT04815356 | Phase I Study of Anti-CD22 Chimeric Receptor T Cells in Patients With Relapsed/Refractory Hairy Cell Leukemia and Variant | Recruiting | Hairy Cell Leukemia|Hairy Cell Leukemia Variant | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1 |
| NCT05651100 | Safety and Efficacy of Sequential CD19 and CD22 Targeted CAR-T Therapy for Relapsed/Refractory B Cell Lymphoma | Not yet recruiting | Lymphoma, B-Cell | Kecellitics Biotech Company Ltd|Hebei Yanda Ludaopei Hospital | Phase 1|Phase 2 |
| NCT04303247 | CD19 and CD22 Dual-targeted CAR-T Cells for Relapsed or Refractory B-NHL | Not yet recruiting | B-cell Lymphoma Refractory|B-cell Lymphoma Recurrent | Xinqiao Hospital of Chongqing|Gracell Biotechnology Shanghai Co., Ltd.|920th Hospital of Joint Logistics Support Force of People's Liberation Army of China|The Affiliated Hospital Of Guizhou Medical University|Tang-Du Hospital|The General Hospital of Western Theater Command|Chongqing University Cancer Hospital | Early Phase 1 |
| NCT04626739 | CAR-T Cells in Treating Patients With Relapsed or Refractory NHL | Recruiting | Refractory Indolent Adult Non-Hodgkin Lymphoma | Hebei Senlang Biotechnology Inc., Ltd. | Early Phase 1 |
| NCT04626726 | Adult B-ALL Treated by CAR-T Cell Bridging Allogeneic Hematopoietic Stem Cell Transplantation | Recruiting | Adult B Acute Lymphoblastic Leukemia | Hebei Senlang Biotechnology Inc., Ltd. | Early Phase 1 |
| NCT05388695 | To Observe the Dual-target Chimeric Antigen Receptor T Cells in the Treatment of B Cell Hematologic Tumors | Recruiting | 19 and 22+ B Cell Hematologic Tumors|19 and 20+ B Cell Hematologic Tumors | Hebei Senlang Biotechnology Inc., Ltd.|Tongji Hospital | Phase 1 |
| NCT04601181 | Safety and Efficacy of ThisCART22 in Patients With Refractory or Relapsed B Cell Malignancies | Recruiting | B Cell Malignancy | Fundamenta Therapeutics, Ltd.|The First Affiliated Hospital of USTC (Anhui Provincial Hospital) | Phase 1 |
| NCT05106946 | Safety and Clinical Activity of ThisCART22 in Patients With r/r Non-Hodgkin's B Cell Lymphoma | Recruiting | B-cell Malignancy | Fundamenta Therapeutics, Ltd.|The Affiliated Hospital of Xuzhou Medical University | Phase 1 |
| NCT04626765 | CAR-T for Children With Relapsed and Refractory Acute Lymphoblastic Leukemia | Recruiting | Childhood Acute Lymphoblastic Leukemia | Hebei Senlang Biotechnology Inc., Ltd.|The Second Hospital of Hebei Medical University | Early Phase 1 |
| NCT04340154 | Study of Sequential CAR-T Cell Treating Leukemia Children | Recruiting | Acute Lymphoblastic Leukemia|Acute Lymphoblastic Leukemia, in Relapse|Refractory Acute Lymphoid Leukemia | Beijing Boren Hospital | Phase 2 |
| NCT05206071 | Clinical Study of SL19+22 CAR-T Cells for Relapsed or Refractory Non-Hodgkin Lymphoma | Recruiting | Non-hodgkin's Lymphoma | Hebei Senlang Biotechnology Inc., Ltd. | Not Applicable |
| NCT04283006 | A Study of CD20/CD22 Targeted CAR T-cell Therapy for Relapsed or Refractory Lymphoid Malignancies | Recruiting | Relapsed and Refractory|Lymphoid Hematological Malignancies | He Huang|Yake Biotechnology Ltd.|Zhejiang University | Early Phase 1 |
| NCT04781634 | a Clinical Research of CD19 and CD22 Targeted Prime CAR-T Cell in Relapsed/Refractory B-ALL | Recruiting | B-ALL | Chongqing Precision Biotech Co., Ltd | Phase 1|Phase 2 |
| NCT04782193 | a Clinical Research of CD19 and CD22 Targeted Prime CAR-T Cell in Relapsed/Refractory B Cell Lymphoma | Recruiting | B Cell Lymphoma | Chongqing Precision Biotech Co., Ltd | Phase 1|Phase 2 |
| NCT05470777 | CD22/CD19 CAR-T and Auto-HSCT Sandwich Strategy as Consolidation Therapy for B-ALL | Recruiting | B-cell Acute Lymphoblastic Leukemia | The First Affiliated Hospital of Soochow University|National Natural Science Foundation of China(Grant No. 81970138)|Jiangsu Province Natural Science Foundation of China (Grant No. BK20210091)|Jining Medical University|The Second People's Hospital of Huai'an|First Affiliated Hospital Bengbu Medical College|Northern Jiangsu Province People's Hospital|Affiliated Hospital of Nantong University|Suzhou Hospital of Traditional Chinese Medicine | Phase 1|Phase 2 |
| NCT04571138 | A Pediatric and Young Adult Trial of Genetically Modified T Cells Directed Against CD22 for Relapsed/Refractory Leukemia or Lymphoma | Recruiting | Leukemia|Lymphoma | Seattle Children's Hospital | Phase 1|Phase 2 |
| NCT04626908 | Clinical Study of Targeting CD19 and CD22 Chimeric Antigen Receptor T Lymphocytes in the Treatment of Recurrent or Refractory B Cell Non-Hodgkin Lymphoma | Not yet recruiting | Relapsed and Refractory|Lymphoid Hematological Malignancies | He Huang|Gracell Biotechnology Ltd.|Zhejiang University | Phase 1|Phase 2 |
| NCT02443831 | CARPALL: Immunotherapy With CD19/22 CAR T-cells for CD19+ Haematological Malignancies | Active, not recruiting | Acute Lymphoblastic Leukemia|Burkitt Lymphoma | University College, London | Phase 1 |
| NCT05429905 | Dual Anti-CD22/CD19 Chimeric Antigen Receptor-directed T Cells (CART2219.1) for Relapsed Refractory B-Lineage Leukaemia | Recruiting | Lymphoblastic Leukemia|Lymphoblastic Leukemia, Acute, Childhood|Lymphoblastic Leukemia in Children|Lymphoblastic Leukemia, Acute Adult|CAR | KK Women's and Children's Hospital|Singapore General Hospital|National University Hospital, Singapore | Phase 1|Phase 2 |
| NCT04204161 | A Clinical Study of CAR-T Cells Treatment for Children With CD19+/CD22+ R/R ALL and Lymphoma | Recruiting | Relapsed B-cell Acute Lymphoblastic Leukemia, Childhood|Refractory B-cell Acute Lymphoblastic Leukemia, Childhood|Relapsed/Refractory B-cell Lymphoma, Childhood | Shenzhen BinDeBio Ltd.|Xiangya Hospital of Central South University | Phase 1 |
| NCT05098613 | Preliminary Safety and Tolerability of CD19x22 CAR-T Cells in Adolescent and Adult R/R B-NHL Patients | Recruiting | Non-Hodgkin Lymphoma | University of Colorado, Denver | Phase 1 |
| NCT03287817 | CD19/22 CAR-T Cells (AUTO3) for the Treatment of Diffuse Large B Cell Lymphoma | Active, not recruiting | Diffuse Large B Cell Lymphoma|Relapsed Diffuse Large B-Cell Lymphoma|Refractory Diffuse Large B-Cell Lymphoma|DLBCL | Autolus Limited | Phase 1|Phase 2 |
| NCT05091541 | A Phase 1/2 Study of CT120 in Patient With Relapsed/Refractory B-cell Non-Hodgkin's Lymphoma | Not yet recruiting | B-cell Non-Hodgkin's Lymphoma | Nanjing IASO Biotherapeutics Co., Ltd | Phase 1|Phase 2 |
| NCT04430530 | 4SCAR-T Therapy Post CD19-targeted Immunotherapy | Recruiting | CD19 Negative B-cell Malignancies | Shenzhen Geno-Immune Medical Institute|ShiJiaZhuang Zhongxi Children Hospital|Shenzhen Children's Hospital|The Seventh Affiliated Hospital of Sun Yat-sen University | Phase 1|Phase 2 |
| NCT04429438 | Multi-CAR-T Cells Targeting B Cell Lymphomas | Recruiting | B Cell Lymphoma (BCL) | Shenzhen Geno-Immune Medical Institute|The Seventh Affiliated Hospital of Sun Yat-sen University|Shenzhen Children's Hospital | Phase 1|Phase 2 |
| NCT04016129 | CAR-T Immunotherapy Targeting CD19- ALL | Recruiting | B-cell Leukemia | Shenzhen Geno-Immune Medical Institute | Phase 1|Phase 2 |
| NCT03407859 | Sequential Treatment With CD20/CD22/CD10-CART After CD19-CART Treatment Base on MRD in Relapsed/Refractory B-ALL | Recruiting | Therapy Related Leukemia | Zhujiang Hospital|Nanfang Hospital of Southern Medical University | Early Phase 1 |
| NCT05432882 | CD19/22 Bi-specific CAR-T Cell Therapy | Recruiting | B Cell Malignancies | Shenzhen Geno-Immune Medical Institute | Phase 1|Phase 2 |
| NCT05010564 | CAR-T Cell, B-cell Acute Lymphoblastic Leukemia (TriCAR) | Not yet recruiting | Leukemia, B-Cell | Baylor College of Medicine|Texas Children's Cancer Center | Phase 1 |
| NCT03614858 | CD19/CD22-targeted Chimeric Antigen Receptor Engineered T Cell (CART) in B-Cell Acute Lymphoblastic Leukemia. | Recruiting | Leukemia, B-cell | Shanghai Unicar-Therapy Bio-medicine Technology Co., Ltd|The First Affiliated Hospital of Soochow University | Phase 1|Phase 2 |
| NCT02650414 | CD22 Redirected Autologous T Cells for ALL | Recruiting | B Cell Leukemias|B Cell Lymphomas | University of Pennsylvania|Children's Hospital of Philadelphia | Phase 1 |
| NCT04150497 | Phase 1/2 Study of UCART22 in Patients With Relapsed or Refractory CD22+ B-cell Acute Lymphoblastic Leukemia (BALLI-01) | Recruiting | B-cell Acute Lymphoblastic Leukemia | Cellectis S.A. | Phase 1 |
| NCT05292898 | A Triple-targeted Cell Preparation Targeting CD19/CD20/CD22 in Patients With Relapsed/Refractory B-cell Acute Lymphocytic Leukemia | Recruiting | Acute Lymphocytic Leukemia | Institute of Hematology & Blood Diseases Hospital|Nanjing Legend Biotech Co. | Phase 1 |
| NCT03620058 | CART22 Alone or in Combination With huCART19 for ALL | Active, not recruiting | Chemotherapy Resistant Acute Lymphoblastic Leukemia|Refractory Acute Lymphoblastic Leukemia | University of Pennsylvania | Phase 1 |
| NCT05318963 | Targeting CD19/CD20/CD22 Triple-targeted Cell in Patients With Relapsed/Refractory B-cell Lymphoma | Recruiting | B-cell Lymphoma Recurrent|B-cell Lymphoma Refractory | Qiu Lugui|Nanjing Legend Biotech Co.|Institute of Hematology & Blood Diseases Hospital | Phase 1 |
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION