Cell and gene therapies (CGT) represent revolutionary medical advances, targeting diseases at their genetic or cellular origins. Unlike traditional therapies that manage symptoms, CGTs aim to correct the underlying cause of diseases by repairing, replacing, or modifying genetic material or cells within the body1. These groundbreaking approaches promise significant improvements in managing, treating, and potentially curing previously incurable diseases.
Cell therapies involve introducing healthy or genetically engineered cells into patients to repair or replace damaged tissues or combat diseases. This approach can either utilize the patient's own cells (tautologous) or cells from a donor (genealogical). CAR-T therapy exemplifies this approach, where a patient's own T cells are collected, genetically modified outside the body to recognize and attack specific cancer cells, and then reinfused2. Other examples include stem cell therapies used in regenerative medicine to repair damaged tissues such as cartilage or heart muscle.
Gene therapy focuses on correcting defective genes by inserting, altering, or removing genetic material. CRISPR-Cas9, a prominent gene-editing tool, has shown great potential in treating sickle cell anaemia by editing genes within hematopoietic stem cells to restore normal function3. Gene therapies can be delivered using viral vectors such as Adenovirus or Adeno-associated viruses (AAV), non-viral delivery systems like lipid nanoparticles, or physical methods. Each method offers unique advantages and is chosen based on specific therapeutic needs and targeted tissues.
Several recent successes highlight the growing clinical viability and therapeutic promise of CGT. One major milestone is the approval of CASGEVY in 2023-the first CRISPR-Cas9 therapy used in humans for sickle cell anaemia. By editing the BCL11A enhancer region in hematopoietic stem cells, the therapy boosts fatal haemoglobin levels and significantly reduces vaso-occlusive crises, providing patients with improved quality of life and, in some cases, a functional cure. Clinical trials have demonstrated sustained remission and improved haemoglobin levels over extended follow-up periods.
Zynteglo, also based on lentiviral gene therapy, offers a therapeutic option for patients with transfusion-dependent Beta-Thalassemia. By enabling these individuals to produce functional haemoglobin, Zynteglo allows many to discontinue chronic transfusions, minimizing iron overload and improving organ health. These breakthroughs underscore the transformative role of gene addition therapies in hematologic conditions.
In the cancer treatment landscape, Breyanzi represents a new-generation CAR-T therapy approved for large B-cell lymphoma. By engineering autologous T cells to express CD19-specific chimeric antigen receptors, Breyanzi targets malignant B cells with high specificity. Compared to earlier CAR-T products, Breyanzi shows more consistent manufacturing, reduced cytokine release syndrome (CRS) rates, and robust efficacy in relapsed or refractory cases.
These therapies mark a shift from experimental to standard-of-care in specific indications. Continued clinical trials are underway to evaluate their long-term durability, safety in broader populations, and potential expansion into earlier lines of treatment or additional disease subtypes. Their success also paves the way for further innovations in in vivo editing, multiplex gene engineering, and immune cell programming.
Several recent successes highlight the potential of CGT. CASGEVY, approved in 2023, became the first CRISPR-Cas9 therapy for sickle cell anaemia, significantly reducing painful crises by boosting fatal haemoglobin production. Zynteglo, another significant therapy, treats transfusion-dependent beta-thalassemia by enabling patients to produce their own functional haemoglobin. Breyanzi, a CAR-T therapy, provides durable remission for large B-cell lymphoma by re-engineering patient T cells to target cancer cells specifically.
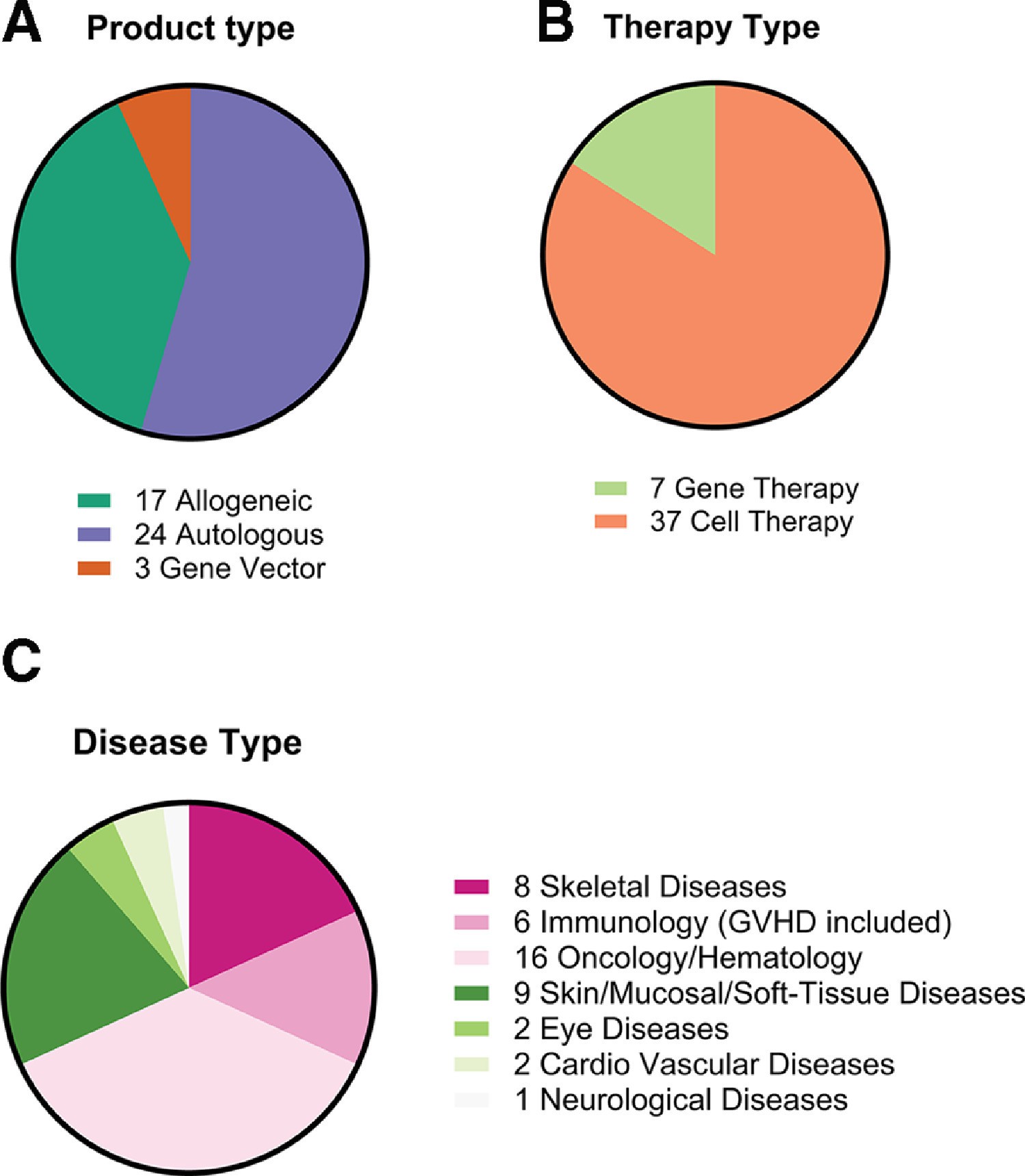 Fig.1 Cell, tissue and gene products with MA worldwide (44 unique products) organized by (A) product type, (B) therapy type and (C) disease type. "Cell Therapy" products in (B) also include tissue engineered products4.
Fig.1 Cell, tissue and gene products with MA worldwide (44 unique products) organized by (A) product type, (B) therapy type and (C) disease type. "Cell Therapy" products in (B) also include tissue engineered products4.
Groundbreaking cases illustrate the developmental impact of CGTs across a wide spectrum of medical conditions and age groups. One of the most striking examples involved a newborn diagnosed with a rare monogenic disorder, who received a personalized CRISPR-Cas9 therapy specifically designed to target and correct the underlying genetic mutation. This pioneering case demonstrated not only the feasibility of individualized genome editing but also the potential of CGTs to provide life-altering results from the earliest stages of life. In another significant case, a four-year-old child with leukocyte adhesion deficiency, an often fatal immunodeficiency disorder, underwent gene therapy that successfully reconstituted normal immune function and enabled the child to live a healthy, infection-free life. Furthermore, a groundbreaking ocular gene therapy restored partial vision to a child born with Leber congenital amaurosis, a severe form of inherited blindness. This case highlighted the potential of gene therapies to treat sensory deficits and offered hope to many suffering from inherited retinal diseases5. Collectively, these cases exemplify how CGTs are not just promising in theory, but are delivering tangible outcomes that reshape the course of patients' lives.
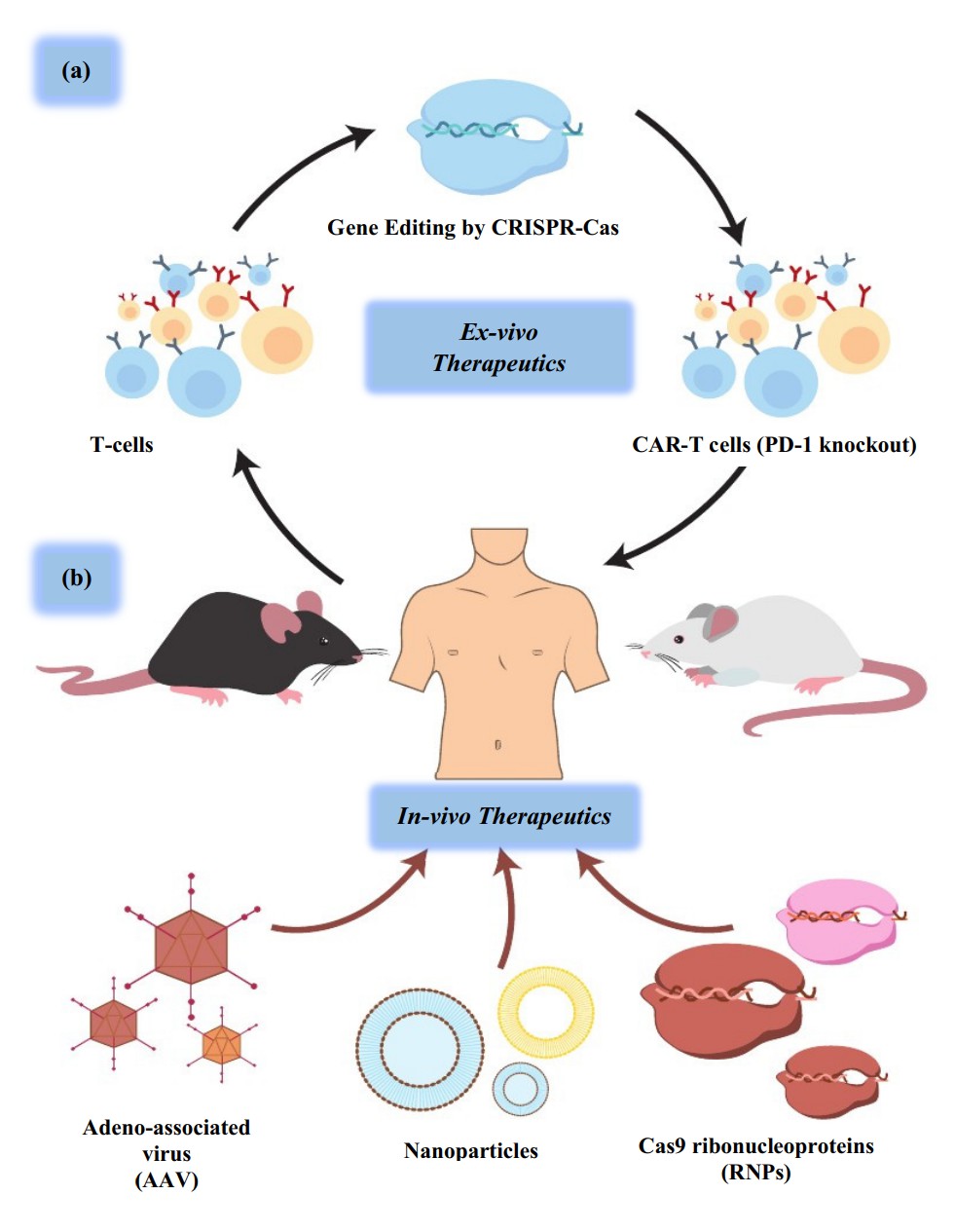 Fig.2 Functions of CRISPR-Cas9 variants6
Fig.2 Functions of CRISPR-Cas9 variants6
The promise of cell and gene therapies is no longer confined to rare monogenic disorders or hematologic malignancies. Increasingly, researchers are exploring the application of CGT platforms to more prevalent and complex conditions, including cardiovascular, neurological, and metabolic diseases.
A notable example is VERV-102, an innovative in vivo base-editing therapy developed by Verve Therapeutics. Unlike traditional gene therapies that rely on viral vectors to insert corrective genes, VERV-102 uses lipid nanoparticles to deliver a base editor that directly modifies the PCSK9 gene in liver cells7. This results in long-lasting reduction in LDL cholesterol levels, addressing a key risk factor for atherosclerotic cardiovascular disease—the leading cause of death globally. Early clinical data in primates and initial human trials have shown significant LDL reductions after a single treatment, potentially replacing lifelong inhibin use.
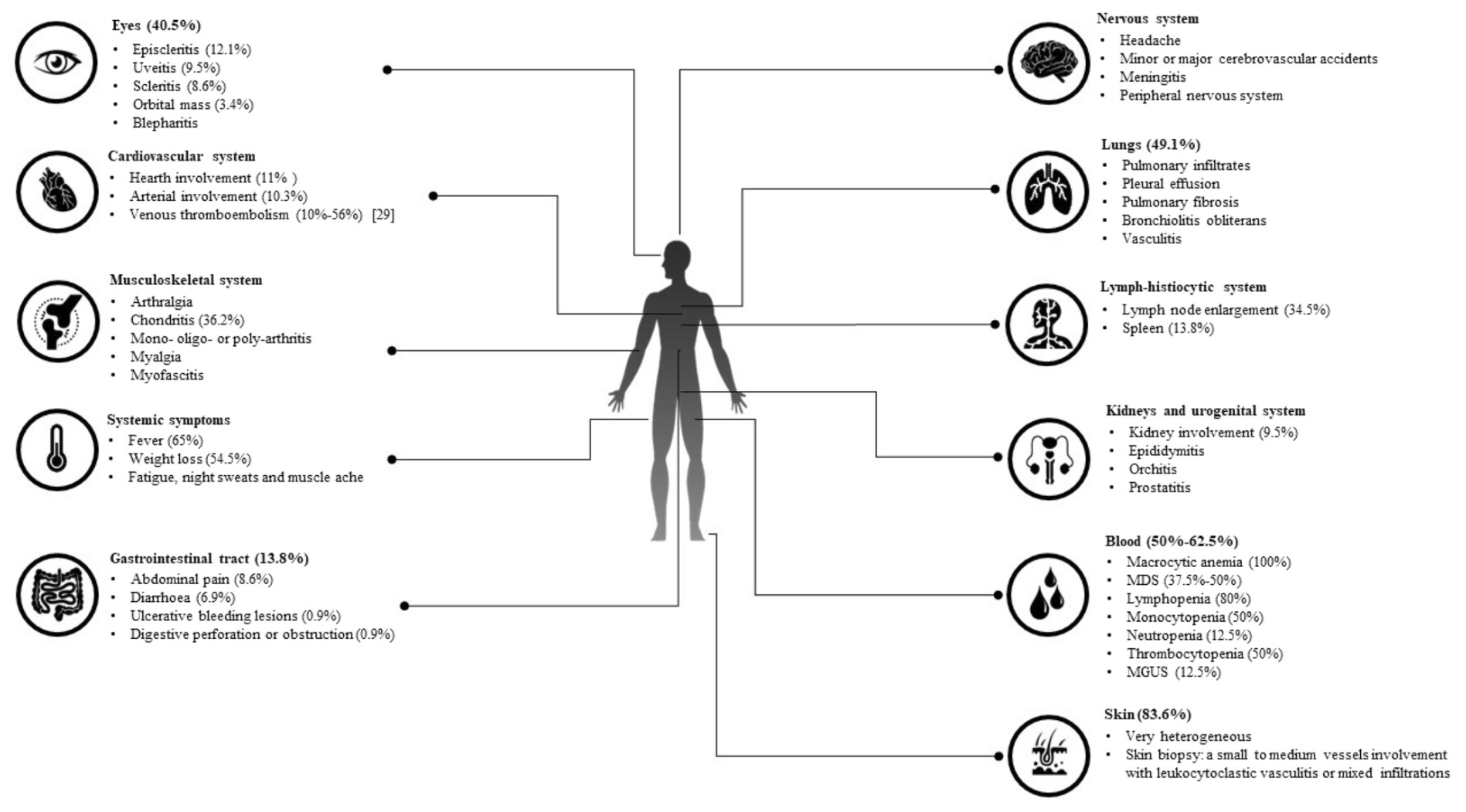 Fig.3 The cartoon describes the main clinical and laboratory haematological manifestations in VEXAS patients8
Fig.3 The cartoon describes the main clinical and laboratory haematological manifestations in VEXAS patients8
Similarly, AVB-101, a gene therapy being developed by AviadoBio, targets frontotemporal dementia (FTD) caused by GRN gene mutations. This therapy delivers a functioning GRN gene directly to neurons in the thalamus using an adeno-associated virus (AAV) vector via MRI-guided neurosurgical infusion. Preclinical stages in mice and nonhuman primates demonstrated robust transgene expression and increased progranulin levels, offering hope for disease modification in a neurodegenerative disorder with no effective treatments. A Phase 1/2 clinical trial is currently underway.
In addition, there is growing interest in using engineered immune cells for autoimmune diseases. For instance, regulatory T cells genetically modified to enhance immunological function are being studied for conditions like Type 1 diabetes and multiple sclerosis. These therapies aim to restore immune tolerance, reduce inflammation, and prevent tissue destruction.
Other pipelines are exploring gene therapy for inherited metabolic disorders like phenylketonuria, as well as muscular dystrophies, age-related macular degeneration, and even acquired conditions such as heart failure. The flexibility of delivery platforms—ranging from viral vectors to non-viral nanoparticles—enables tailored approaches for various tissues and diseases.
These expanding applications illustrate how CGT is evolving from niche treatments into mainstream therapeutic strategies, potentially transforming the standard of care across multiple disciplines.
Emerging therapies extend CGT's reach beyond rare genetic disorders into common chronic diseases. VERV-102, developed by Verve Therapeutics, uses base-editing technology delivered via lipid nanoparticles to permanently lower cholesterol, representing a potential breakthrough in cardiovascular disease management. AviadoBio's AVB-101 aims to address dementia by delivering corrective genes directly into the brain, showing promise in ongoing clinical trials.
Manufacturing scalability remains a critical challenge for CGTs, particularly for autologous cell therapies that require individualized processing of each patient's cells. This process includes harvesting, genetic modification, quality control testing, and re-administration, which is time-consuming, labour-intensive, and prone to variability. In contrast, allogeneic approaches, where cells from a single donor can be expanded and used for multiple patients, promise greater efficiency but bring challenges in immune compatibility and long-term safety.
To address these challenges, advancements in processing technologies are being developed, including closed-system manufacturing, automation platforms, and artificial intelligence for real-time process monitoring. For example, automated cell culture and fill-finish systems are reducing human error while improving throughput. Moreover, the integration of Good Manufacturing Practice (GMP) facilities with modular or mobile manufacturing units could enhance accessibility in both centralized and decentralized clinical settings.
Regulatory bodies like the FDA and EMA are also adapting to the fast pace of CGT innovation by offering accelerated approval pathways and guidance for Chemistry, Manufacturing and Controls (CMC) documentation. Nonetheless, harmonization across jurisdictions remains an area requiring further work. Improvements in automation, standardization, supply chain logistics, and international regulatory convergence are vital for broader application, commercial scalability, and affordability of CGTs.
The rapid development of CGT raises significant ethical concerns, including genetic privacy, informed consent, equity in access, and potential unintended genetic modifications. Responsible governance, comprehensive ethical frameworks, and public engagement are necessary to navigate these complex issues, ensuring these powerful technologies benefit all of society responsibly.
Future research in CGT is directed toward enhancing delivery systems, increasing specificity and safety of gene-editing tools, and expanding therapeutic applications. Novel vectors, improved gene-editing precision, and better understanding of cellular mechanisms will further advance these therapies. Global collaboration and knowledge-sharing between researchers, clinicians, industry, and regulators will accelerate development and implementation9.
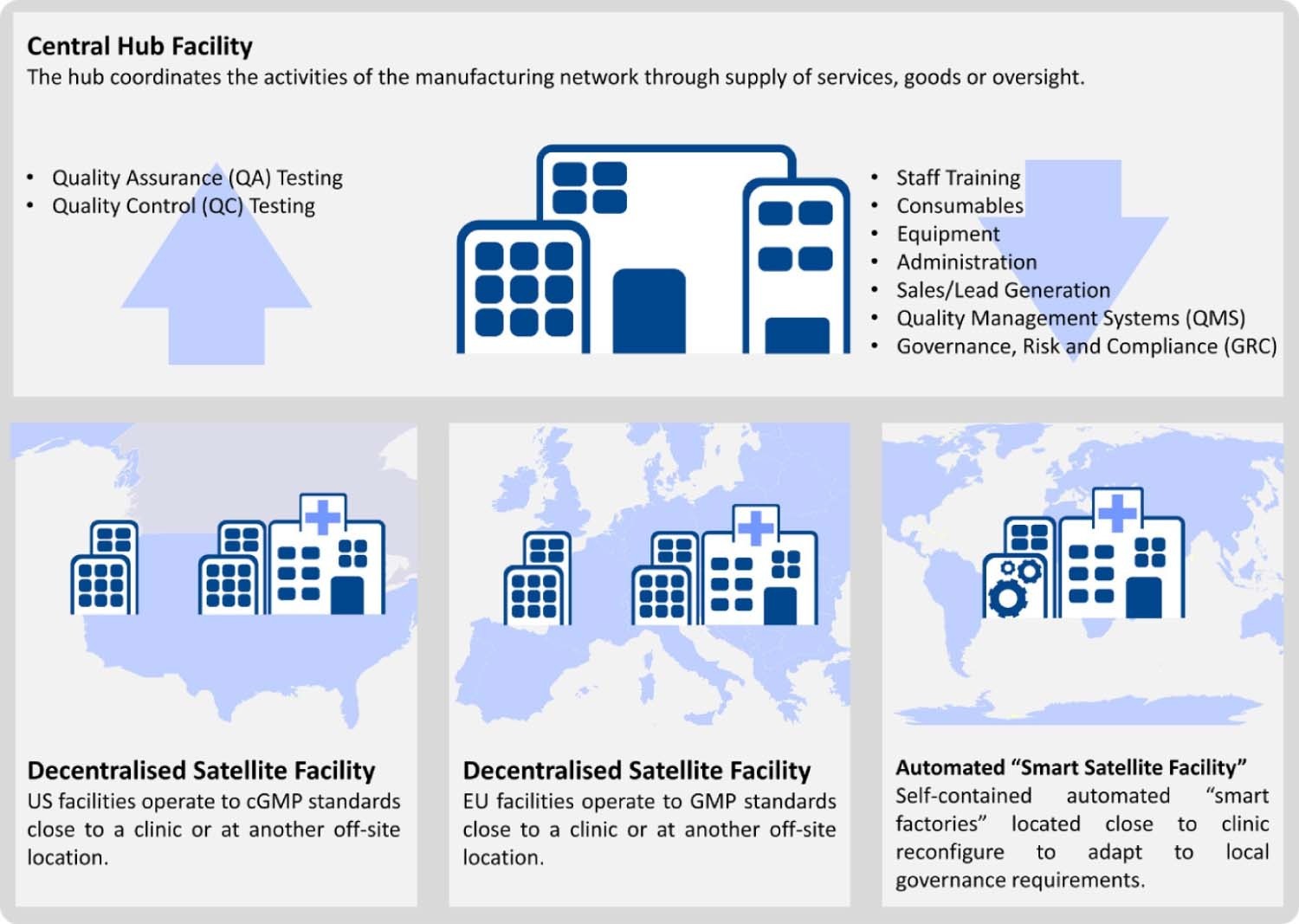 Fig.4 Decentralized manufacturing strategies for CGTs. Satellite facilities may operate in stand-alone locations or close-to-clinic. The automated "smart satellite facility" forgoes the complexities of a large staffed facility, instead externalizing and automating processes and services to reduce the geographic pressures that affect facility location10
Fig.4 Decentralized manufacturing strategies for CGTs. Satellite facilities may operate in stand-alone locations or close-to-clinic. The automated "smart satellite facility" forgoes the complexities of a large staffed facility, instead externalizing and automating processes and services to reduce the geographic pressures that affect facility location10
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION