The field of Cell and Gene Therapy is built upon decades of advancements in molecular biology, immunology, and genetics, culminating in a class of therapies that treat disease by repairing, replacing, or reprogramming the body's own cells and genetic code. This section provides a detailed overview of the scientific principles that underpin these transformative treatments, charting their evolution from theoretical concepts to approved, life-altering medicines.
The articles gathered here define and differentiate the primary modalities of CGT. They explain the mechanisms of cell therapies, such as Chimeric Antigen Receptor (CAR) T-cell immunotherapy, where a patient's own T-cells are engineered to recognize and eliminate cancer cells. They also demystify gene therapies, which focus on correcting defective genes through various strategies, including the use of viral vectors like adeno-associated virus (AAV) to deliver functional gene copies, or the application of precise gene-editing tools like CRISPR-Cas9 to modify the genome directly. This collection chronicles the landmark clinical successes that have validated these approaches while providing a sober assessment of the remaining scientific, safety, and ethical challenges that the field must continue to address.
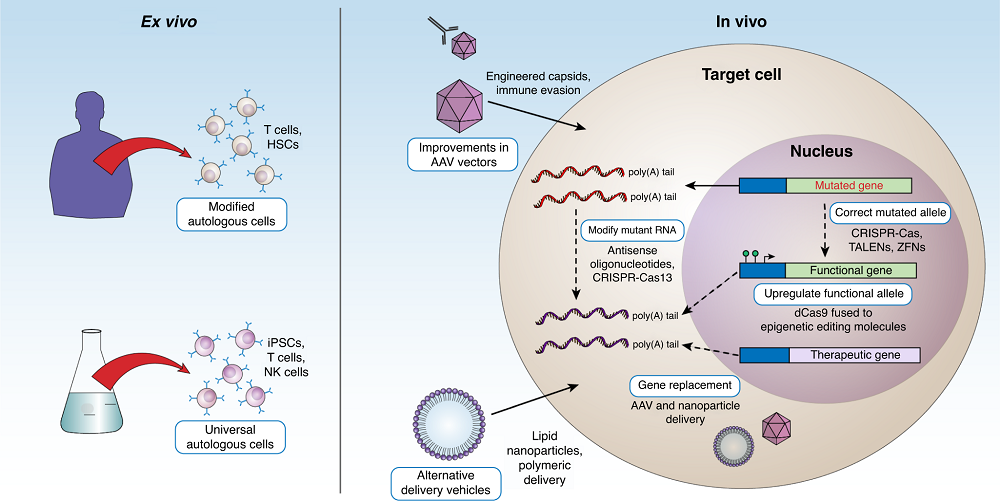 Fig.1 Ex vivo and in vivo strategies for treating genetic diseases1,2
Fig.1 Ex vivo and in vivo strategies for treating genetic diseases1,2
This article provides a foundational introduction to the core concepts of CGT, distinguishing between cell-based approaches that introduce healthy or engineered cells to repair tissues or combat disease, and gene-based strategies that correct underlying genetic defects. It contextualizes these principles with detailed discussions of recent clinical breakthroughs. These include CASGEVY, the first approved CRISPR-Cas9 therapy, which edits the BCL11A enhancer in hematopoietic stem cells to treat sickle cell anemia, and Breyanzi, a next-generation CAR-T therapy approved for large B-cell lymphoma that demonstrates high specificity in targeting malignant B-cells. The text also explores expanding applications into more prevalent conditions, such as VERV-102, an in-vivo base-editing therapy designed to permanently lower cholesterol.
This piece examines the regulatory classification of CGTs as "Advanced Therapeutic Drugs" and details the significant progress within China's CGT industry, which now operates on par with international standards. It highlights the profound clinical potential of CGT through compelling case studies. Among them is the world's first successful use of CRISPR/Cas9-edited hematopoietic stem cells (knocking out the CCR5 gene) to treat a patient with both AIDS and acute lymphoblastic leukemia. Another case details a novel CAR-T therapy (CARv3-TEAM-E) that demonstrated rapid and dramatic efficacy against recurrent glioblastoma, a historically intractable solid tumor. The article also provides a clear overview of the CGT industry chain, from upstream raw materials to downstream clinical application markets.
Presenting a balanced analysis of the CGT field, this article details both its revolutionary successes and its persistent challenges. It explains the mechanisms of pillar technologies, contrasting cell therapy, which provides "ready-made soldiers," with gene therapy, which provides the "blueprint for repair". The piece then chronicles major clinical achievements, such as CAR-T therapies that have achieved high remission rates in B-cell malignancies and gene therapies like LUXTURNA, which restores vision by delivering a normal RPE65 gene to retinal cells. This is balanced with a technical discussion of key risks, including the potential for off-target effects from CRISPR-Cas9, immunogenicity triggered by viral vectors, and the risk of cytokine release syndrome in CAR-T patients.
This article provides a comprehensive overview of the entire CGT value chain, from basic research to clinical implementation and patient access. It reviews the latest research progress in key technology platforms, including CAR-T cell therapy, which is now being explored for autoimmune diseases, and the CRISPR/Cas9 system, which has been successfully used to treat hereditary blindness. The text explains the essential role of Contract Research Organizations (CROs) as "accelerators" that provide services from target gene screening to toxicology studies. Finally, it analyzes new frameworks for patient access, detailing the U.S. Centers for Medicare & Medicaid Services (CMS) CGT Access Model, which uses outcomes-based agreements to manage the high cost of therapies for diseases like sickle cell anemia.
This forward-looking analysis projects the technological and clinical evolution of cell therapy toward 2030, focusing on innovations designed to overcome current limitations. A key breakthrough discussed is allogeneic "universal" cell therapy (such as UCAR-T), which utilizes CRISPR-Cas9 gene editing to knock out HLA and TCR loci, thereby reducing host rejection and the risk of graft-versus-host disease (GVHD). The text also covers the industrialization of stem cell production through technologies like 3D bioreactors, which can increase cell yield by 15 times, and the development of next-generation solutions like in-vivo cell reprogramming and AI-driven predictive models to personalize treatment plans and forecast patient responses.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION