At the forefront of medical development, cell and gene therapy (CGT) is rewriting the rules of disease treatment at an unprecedented rate. By repairing defective genes, modifying immune cells or replacing damaged tissues, these innovative therapies have brought hope for the cure of many diseases that were once considered terminal. Currently, global research efforts are focused on overcoming technological bottlenecks to move this field from the laboratory to the clinic, ushering in a new era of precision medicine.
Cell therapy and gene therapy represent the two pillars of modern biomedical interventions, with the core difference being the target and mechanism of action. The core of cell therapy is the introduction of living cells with therapeutic properties into the patient's body as "biopharmaceuticals". These cells may be derived from the patient's own body (autologous therapy) or from a healthy donor (allogeneic therapy), and are isolated, expanded, activated, or genetically engineered in vitro to confer specific capabilities. For example, in Chimeric Antigen Receptor T-cell therapy (CAR-T), patient T-cells are modified to accurately recognize and kill cancer cells, while mesenchymal stem cells use their multidirectional differentiation potential and immunomodulatory functions to promote tissue repair. The essence is to introduce a functional cell population that directly replaces damaged cells, performs effector functions, or regulates the microenvironment1.
Gene therapy, on the other hand, focuses on correcting, supplementing, or modulating the genetic material within the patient's cells. The core strategy is to deliver therapeutic nucleic acid sequences (e.g., normal gene copies, gene editing tools, RNA molecules, etc.) into the target cell, with the aim of repairing disease-causing mutations, compensating for defective gene function, silencing deleterious gene expression, or introducing novel therapeutic genes2. Adeno-associated viral vectors are commonly used to deliver gene replacement therapies, while gene editing technologies such as CRISPR-Cas9 enable precise modification of specific sequences in the genome. In short, cell therapy provides the "ready-made soldier or engineer", while gene therapy provides the "blueprint or tool for repair".
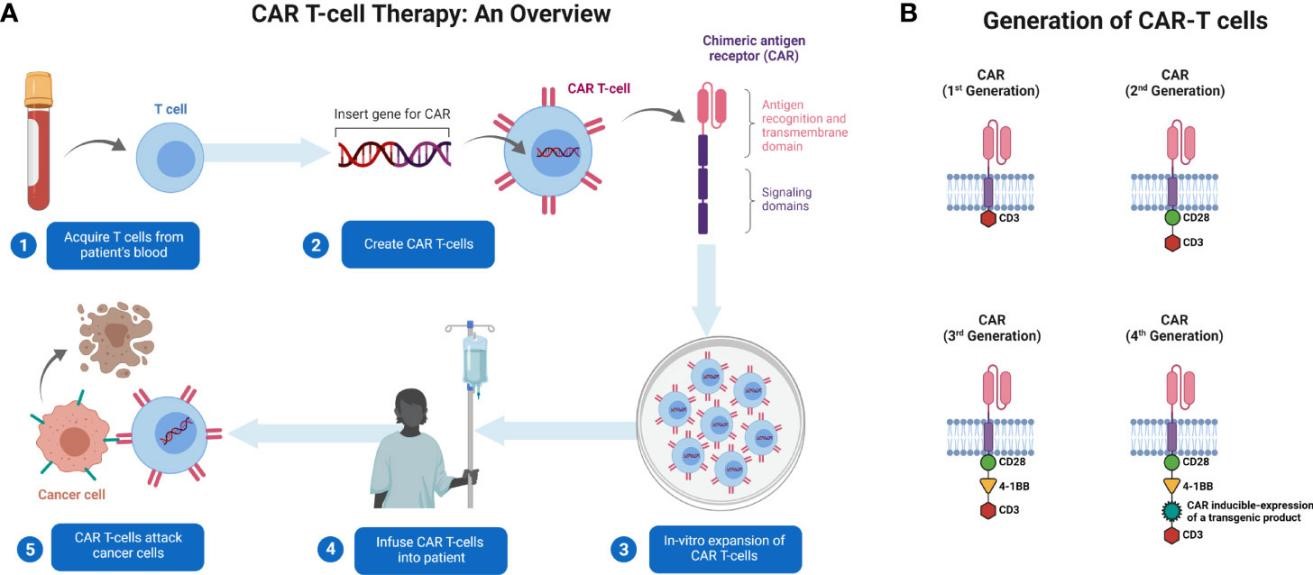 Fig.1 Generation and administration of CAR T-cells in patients with cancer3.
Fig.1 Generation and administration of CAR T-cells in patients with cancer3.
In the field of clinical translation, cell and gene therapy has realized a revolutionary leap from the laboratory to the bedside, bringing hope for the cure of "incurable diseases" that were difficult to overcome in the past. CAR-T cell therapy has made a milestone breakthrough in the treatment of hematological malignant tumors. The patient's own T-cells are genetically engineered to express chimeric receptors that can recognize tumor-specific antigens, which can efficiently eliminate tumor cells after infusion4. CAR-T therapy targeting CD19 has demonstrated a high remission rate in relapsed/refractory B-cell acute lymphoblastic leukemia and certain lymphomas, resulting in long-term disease-free survival or even functional cure for some patients.
Gene therapy has created a paradigm of "one treatment, potentially a cure" in the field of inherited diseases. LUXTURNA, the first FDA-approved in vivo gene therapy, has successfully restored vision to a subset of patients with inherited retinopathies by delivering the normal RPE65 gene to retinal pigment epithelial cells via adeno-associated viral vectors. LUXTURNA has successfully restored vision to some patients with inherited retinopathy.
Zolgensma has significantly improved the motor function and survival of infants with spinal muscular atrophy through intravenous infusion of the AAV9 vector, which carries the functional SMN1 gene, and has altered the natural course of this fatal genetic disease.
In addition, the successful application of stem cell therapy in tissue repair and regeneration, and gene editing therapy in sickle cell disease, demonstrate the potential of such therapies to rewrite the landscape of disease treatment.
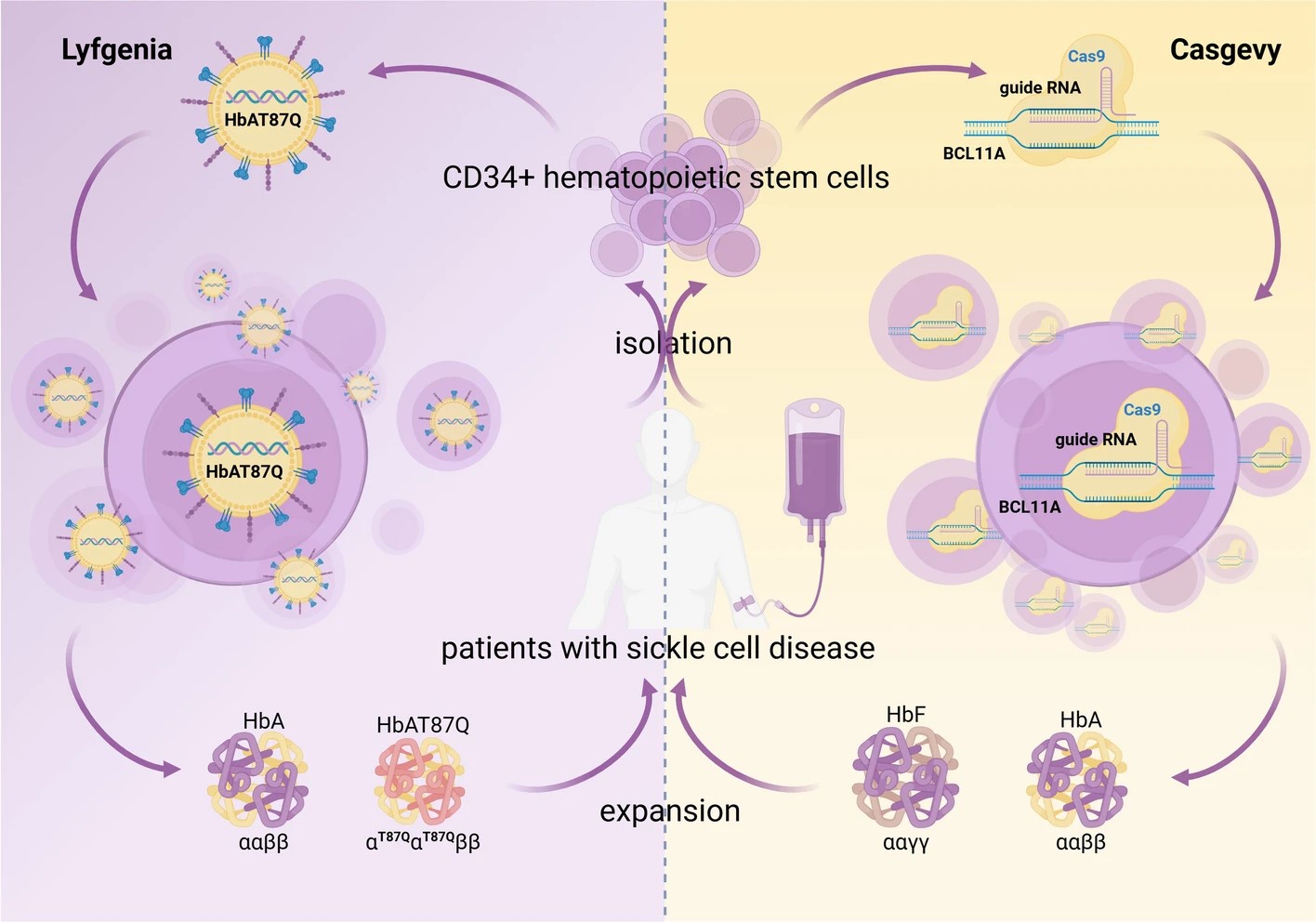 Fig.2 Gene therapy for polygenic or complex diseases2
Fig.2 Gene therapy for polygenic or complex diseases2
The continuous iteration of technology is the core engine driving the rapid development of the cell and gene therapy field. The emergence of gene editing technologies, particularly the CRISPR-Cas system, has enabled unprecedented precision, efficiency and ease of genome manipulation. The CRISPR-based toolbox continues to expand, including base editing to directly convert specific bases without cutting DNA double strands, and lead editing to realize any type of targeted small fragment DNA rewriting, which has dramatically improved the safety and expanded the scope of gene modification.
The innovation of delivery system is the key to break through the bottleneck of in vivo application. As for viral vectors, adeno-associated virus has become the main force of in vivo gene delivery due to its low immunogenicity, long-term expression and tissue-specific serotypes (e.g., AAV9 targeting the nervous system), while lentiviral vectors have been widely used for in vitro gene modification of cells.
To overcome the limitations of viral vectors, non-viral delivery systems are developing rapidly, such as lipid nanoparticles in mRNA vaccines, which have proven to be highly efficient, and novel polymer nanoparticles, exosomes, and physical delivery methods are being explored to improve targeting, loading, and reduce toxicity.
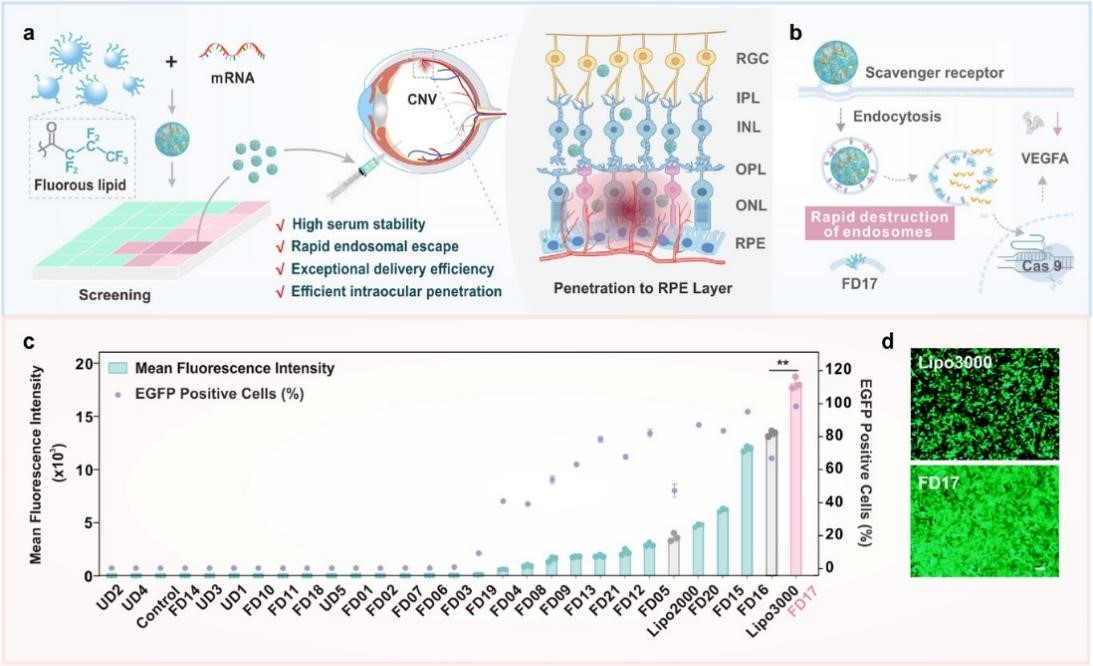 Fig.3 Screening of fluoropolymers for intracellular mRNA delivery and gene editing in the treatment of AMD5
Fig.3 Screening of fluoropolymers for intracellular mRNA delivery and gene editing in the treatment of AMD5
Behind the breakthrough efficacy, safety challenges are always the overhanging sword. The off-target effects of gene editing are a primary concern, as nucleases such as CRISPR-Cas9 may incorrectly cleave non-targeted sites in the genome, leading to unintended mutations and potential cancer risks. Improved high-fidelity enzyme variants, optimized guide RNA design algorithms, and advances in off-target detection technologies can help with risk control. Immunogenicity is another major concern. Viral vectors can trigger a strong immune response, leading to vector clearance, target cell damage or limiting repeat administration; therapeutic transgene products may also be recognized as exogenous antigens.
Excessive immune activation in CAR-T therapies may trigger life-threatening cytokine release syndrome or neurotoxicity. Long-term safety and efficacy remain questionable: AAV vector-mediated gene expression may decline over time; integrated vectors are at risk for insertional mutagenesis; and the ability of CAR-T cells to persistently expand in vivo and relapse due to tumor immune escape remain to be addressed. Rigorous long-term follow-up studies and innovative safety switch design are the solutions.
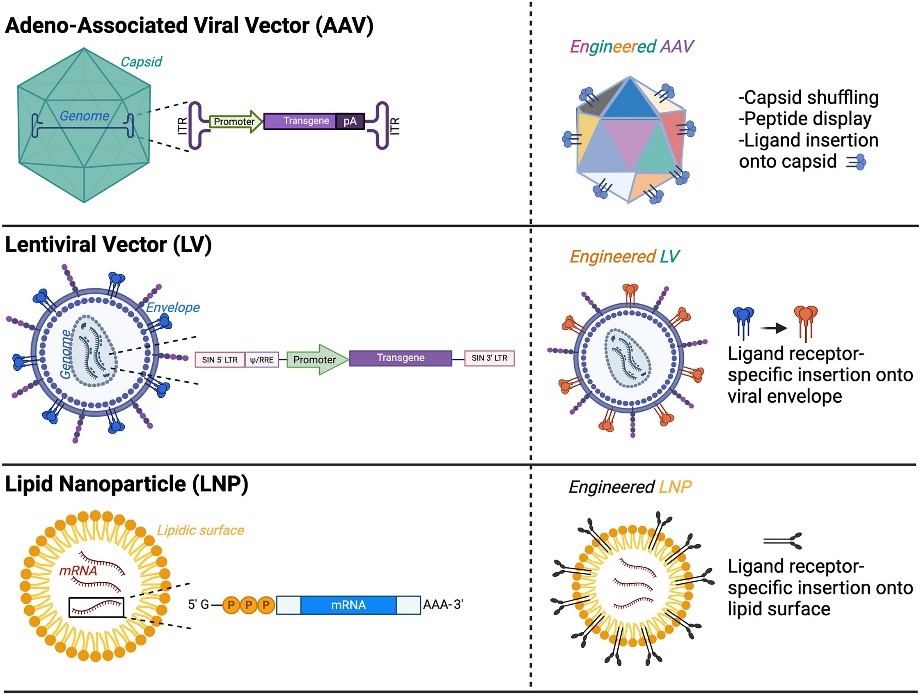 Fig.4 Engineering strategies to enhance the efficacy and safety of gene-therapy vectors6
Fig.4 Engineering strategies to enhance the efficacy and safety of gene-therapy vectors6
Rapid advances in technology continue to challenge the boundaries of ethical and regulatory frameworks. Although somatic cell editing is less controversial, the ethical boundaries, equitable access and potential social impacts of enhanced applications still need to be carefully explored. Cell and gene therapy products are highly complex, individually differentiated and have specific mechanisms of action, making it difficult to fully apply the traditional drug evaluation system. The U.S. FDA's regenerative medicine advanced therapy designation and the European Union's advanced therapeutic drug product regulations are exploring risk-based hierarchical regulation, flexible clinical endpoints and accelerated approval pathways. Establishing globally harmonized regulatory standards, improving long-term safety monitoring systems, and ensuring real-world data collection are the core tasks of regulatory agencies.
The field of cell and gene therapy is seeking breakthroughs with unprecedented vigor, moving toward broader application prospects. Enhancing safety and efficacy is the core direction of attack. The new generation of gene editing tools is pursuing the goal of "zero off-target"; intelligent and adjustable CAR-T design aims at precise activation and reduction of toxicity; and the development of new vectors focuses on tissue targeting, reduction of immunogenicity, and enhancement of expression durability.
Solid tumors are the main focus of CAR-T therapy, and inhibition of the tumor microenvironment and search for better targets are the key; gene therapy is moving towards a wider range of genetic diseases (e.g., Duchenne muscular dystrophy, Huntington's disease), neurodegenerative diseases, cardiovascular diseases, and infectious diseases (e.g., functional cure for HIV). Individualization and precision are the future, and personalized dosing regimens combined with biomarker guidance will maximize therapeutic benefits. Reducing costs and increasing accessibility are the cornerstones of sustainable development.
Cell and gene therapy is leading medicine into a new era where it can directly rewrite the code of life and cellular functions. From curing genetic diseases once thought to be incurable to rearming immune cells to fight cancer, the breakthroughs are exciting. However, the path of scientific discovery must always be underpinned by rigorous safety assessment, ethical reflection, accessibility and forward-looking regulation. Only through collaborative efforts to overcome technological and translational bottlenecks can the infinite possibilities of precision medicine be realized.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION