Cell and gene therapies (CGTs) represent a transformative shift in medicine, directly targeting genetic and cellular origins of disease. Bringing these sophisticated therapies to market demands specialized manufacturing capabilities, rigorous regulatory compliance, and high-quality control processes. This necessity has driven the rise of Contract Development and Manufacturing Organizations (CDMOs), specialized partners essential in translating CGTs from laboratory innovation to commercial-scale production1. CDMOs play a pivotal role in ensuring CGTs' effective commercialization, scalability, and timely delivery to patients.
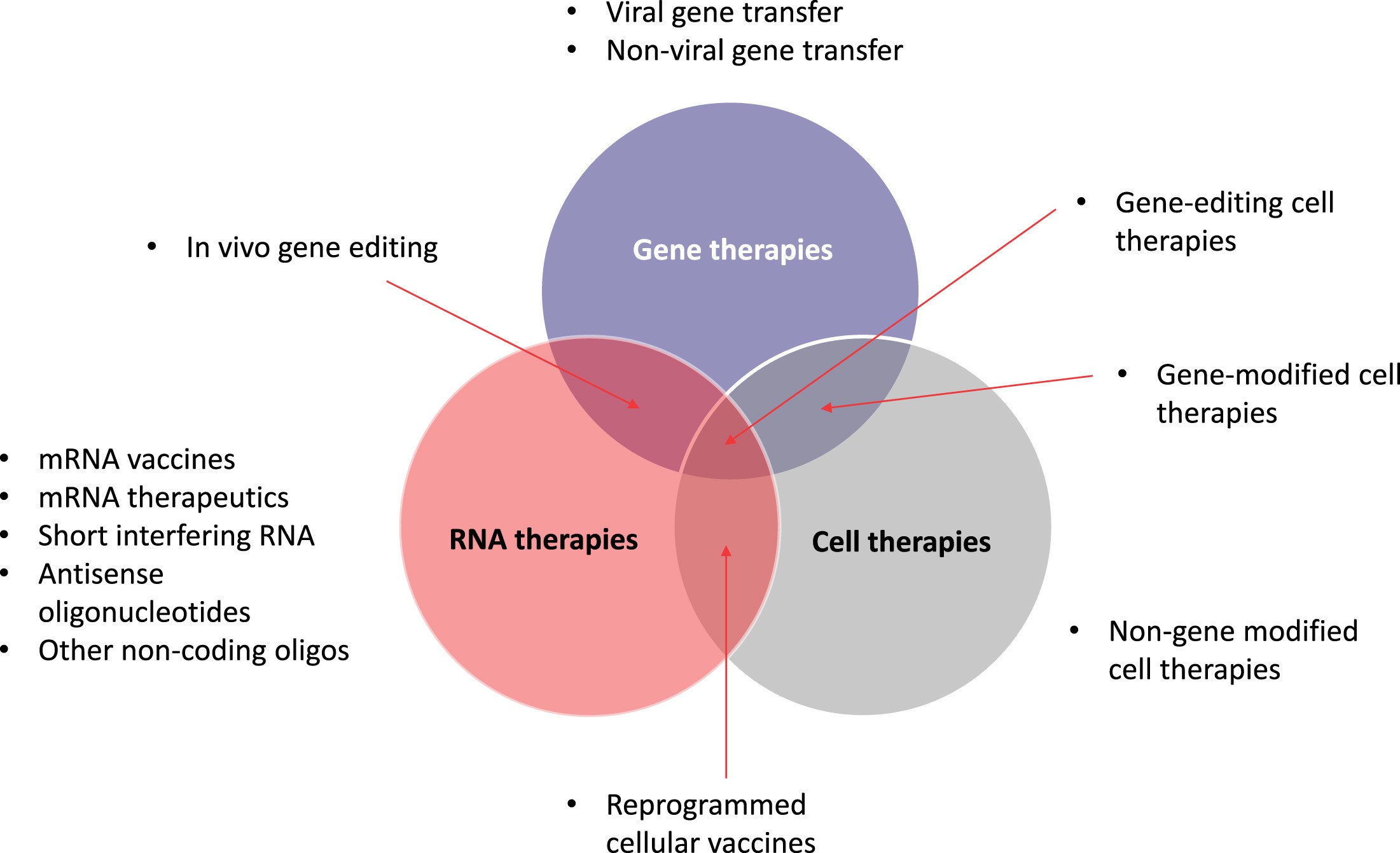 Fig.1 Current universe of advanced therapies, including areas of overlap2
Fig.1 Current universe of advanced therapies, including areas of overlap2
A Contract Development and Manufacturing Organization (CDMO) offers pharmaceutical and biotechnology companies a full suite of development and production services, covering everything from early-stage research support to final commercial-scale manufacturing. These services typically include formulation development, cell and viral vector processing, upstream and downstream process development, GMP-compliant manufacturing, fill-finish operations, packaging, and distribution. In the context of cell and gene therapies (CGTs), these services are uniquely adapted to meet the specific needs of personalized medicine, which requires rigorous aseptic conditions, real-time quality monitoring, and fast turnaround times3.
By outsourcing these highly specialized operations to experienced CDMOs, developers can accelerate their development timelines, access advanced manufacturing infrastructure without the burden of capital investment, and reduce the risk associated with scaling complex biological agents. CDMOs also provide essential regulatory expertise that facilitates faster and smoother submission of Investigational New Drug (IND) applications, Biologics License Applications (BLAs), and other critical documentation. As a result, drug sponsors are able to focus more effectively on innovation, clinical research, and patient outcomes while leveraging the technical and operational capabilities of trusted manufacturing partners.
A contract development and manufacturing Organization (CDMO) is a corporation that provides drug development and manufacturing services to the pharmaceutical sector. CDMOs and pharmaceutical corporations collaborate to outsource medication development and manufacture. CDMOs that provide full service can handle all aspects of drug development and manufacture, as well as work with clients who want to outsource specific parts of their process. It all depends on the requirements of each client. Services which are offered by CDMO comprised of preformulating and formulation development, method development and stability studies, Phase I, and last stage clinical trials, formal stability and scale-up, commercial production, and registration batches, serialization, and shipment, etc.
CDMO differs from traditional drug manufacturer (TDM) and must be considered with respect on those special differences with TDM when GMP and digitalization development are under development and implementation. Briefly, the main aspects of differences between TDM and CDMO are presented on Fig.2. Some of the aspects are discussed below as well.
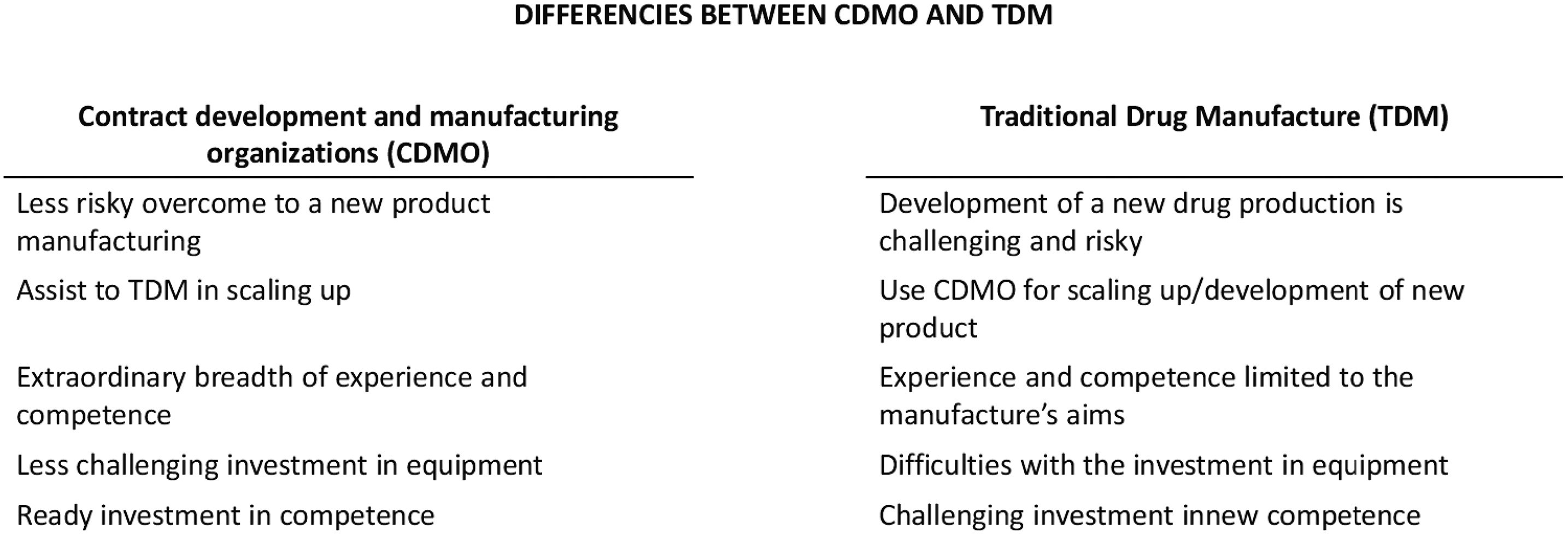 Fig.2 Main different aspects between CDMO and TDM4
Fig.2 Main different aspects between CDMO and TDM4
The development and commercialization of cell and gene therapies face numerous technical and operational complexities. These therapies often involve the use of patient-specific cells or genetically engineered products that require precise handling, sterile environments, and specialized knowledge. CDMOs that focus on CGT have emerged as crucial partners in overcoming these challenges, offering a suite of tailored capabilities that address the unique demands of these advanced therapies. Their involvement extends from early process development to commercial production, ensuring not only efficiency but also regulatory compliance across all stages.
One key advantage of CGT CDMOs is their ability to provide specialized expertise in the handling and manipulation of sensitive biological materials, including viral vectors, genetically modified cells, and stem cells. Their facilities are designed with state-of-the-art infrastructure such as ISO-class cleanrooms and dedicated suites for upstream and downstream bio-processing, supporting the high-purity requirements of CGT workflows. In addition to technical capacity, these CDMOs offer deep regulatory experience, helping navigate complex global approval pathways such as the FDA's RMAT designation and the EMA's Advanced Therapy Medicinal Products framework. Moreover, their scalability ensures seamless transition from small-scale clinical batches to full commercial production, allowing developers to respond swiftly to growing patient demand and commercial opportunities.
CDMOs provide a wide range of essential services that are critical for the successful development and commercialization of advanced therapies. One of the most important is process development, which involves optimizing manufacturing workflows to ensure they are efficient, reproducible, and compliant with regulatory requirements. This includes developing protocols for cell expansion, viral vector production, purification, and formulation. Robust process development not only ensures product consistency but also supports scalability from early clinical trials to commercial production. Analytical method development is another crucial service. CDMOs design and validate precise analytical techniques for assessing product quality, identity, purity, potency, and safety. These methods are essential for both in-process monitoring and lot release testing, playing a key role in regulatory compliance and risk management.
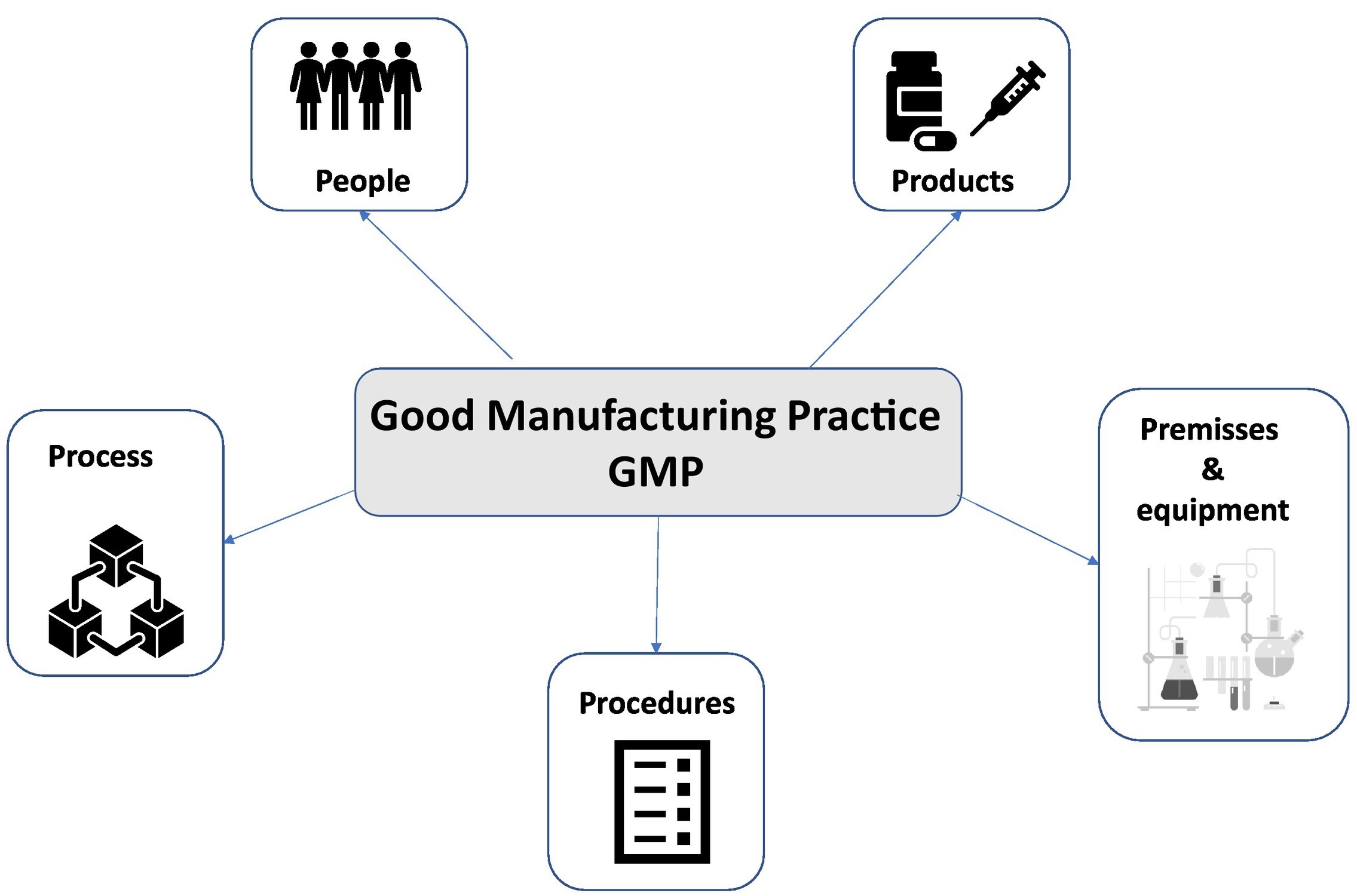 Fig.3 Five principles of Good Manufacturing Practice, schematical overview4
Fig.3 Five principles of Good Manufacturing Practice, schematical overview4
In addition to upstream and downstream process support, CDMOs handle the actual production of CGT products under current Good Manufacturing Practice (cGMP) guidelines. This ensures that all clinical and commercial batches meet stringent quality standards, a particularly demanding task given the sensitive nature of cell and gene-based materials. Regulatory submission support is another indispensable offering; CDMOs assist sponsors in compiling Chemistry, Manufacturing, and Control (CMC) sections for INDs, BLAs, and other submissions. Their regulatory teams provide insight into agency expectations, helping reduce review cycles and approval timelines. Finally, CDMOs play a central role in managing complex supply chains. Given the perishability and temperature sensitivity of many CGT products, CDMOs must maintain highly coordinated logistics, including cold-chain distribution, real-time tracking, and contingency planning for international shipping.
Despite their importance, CGT CDMOs face several significant challenges that can hinder the pace of therapy development and commercialization. One of the most pressing issues is managing complex supply chains. The transport of cell and gene therapy products often requires ultra-cold conditions and tight timing, with even minor delays posing serious risks to product integrity and patient outcomes. The global nature of clinical trials and therapy distribution further complicates logistics, requiring highly coordinated efforts and investment in reliable, validated cold-chain systems. In addition, CDMOs must deal with increasing capacity constraints as the demand for CGT manufacturing outpaces available facilities and workforce. This can result in long lead times, delays in project initiation, and difficulties in scaling manufacturing to meet clinical and commercial needs.
Another substantial challenge lies in navigating diverse and continuously evolving regulatory landscapes. Each geographic region may have unique expectations for documentation, quality control, and product release testing. As such, CDMOs must maintain up-to-date knowledge and maintain flexibility to accommodate different regulatory frameworks while ensuring global compliance. Finally, cost management remains a critical concern. The complex, labour-intensive processes involved in CGT manufacturing often result in high production costs, making therapies less accessible to patients. Efficient process optimization, yield improvement, and automation strategies are crucial to drive down costs without compromising product quality or safety.
The future of CGT CDMOs appears promising with increasing global demand and technological advancements. Enhanced automation, sophisticated analytical methods, and robust regulatory frameworks will streamline CGT production and commercialization. The growing emphasis on strategic partnerships among CDMOs, biotech firms, and regulatory bodies will further support innovation, improve accessibility, and expand the market reach of CGTs globally5.
CDMOs specializing in cell and gene therapies play a critical role in bringing innovative therapies from laboratory research to market readiness. Their specialized expertise, advanced manufacturing capabilities, and regulatory acumen are fundamental in overcoming the challenges inherent in CGT production. As the sector continues to evolve, CGT CDMOs will undoubtedly remain pivotal in transforming groundbreaking scientific advancements into accessible and effective medical treatments worldwide.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION