The translation of a cell or gene therapy from a laboratory protocol into a safe, consistent, and scalable medicinal product is an endeavor of immense technical, logistical, and financial complexity. Unlike conventional pharmaceuticals, CGTs are "living drugs," characterized by inherent biological variability of the starting material and the absolute requirement for aseptic processing from start to finish, as they cannot be terminally sterilized. This reality demands a fundamentally different approach to production, quality control, and distribution, governed by stringent regulatory frameworks.
This section provides a detailed examination of the CGT production and commercialization pathway. The articles herein deconstruct the intricate, multi-stage manufacturing processes and explain the non-negotiable role of Good Manufacturing Practice (GMP) in ensuring product safety and quality. They also analyze the critical function of Contract Development and Manufacturing Organizations (CDMOs) as essential partners that provide the specialized expertise and infrastructure needed in this ecosystem. Furthermore, this collection confronts the significant economic pressures on the healthcare system, detailing the innovative payment and reimbursement models being implemented globally to ensure these high-value therapies are accessible to patients who need them.
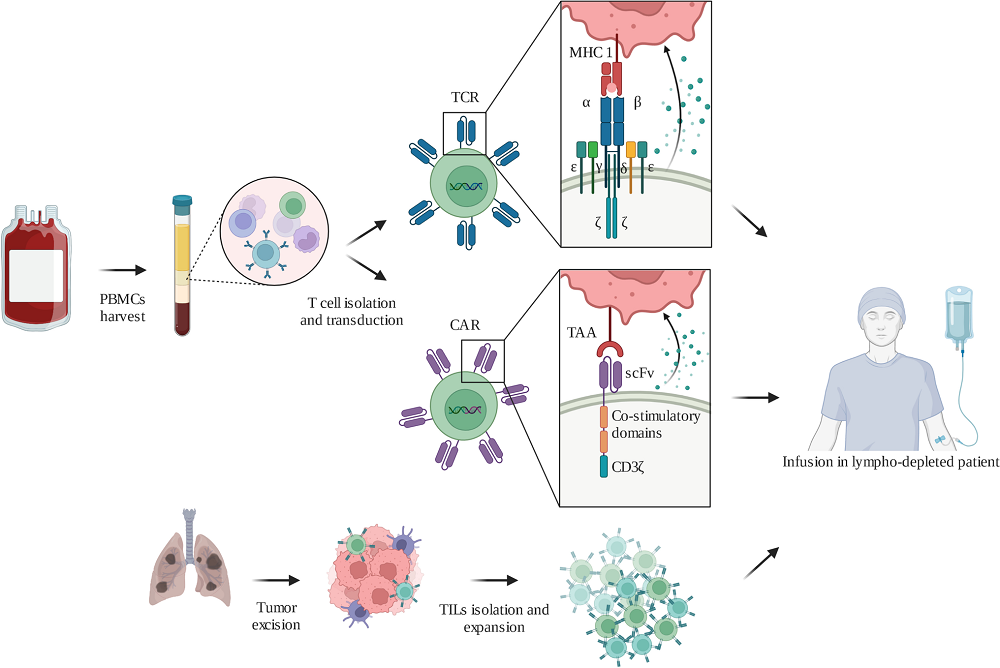 Fig.1 Adoptive cell therapies manufacturing process1,2
Fig.1 Adoptive cell therapies manufacturing process1,2
This article establishes Good Manufacturing Practice (GMP) as the essential regulatory framework that enables the safe clinical use of cell therapies. It defines GMP as a strict set of guidelines ensuring the safety, quality, and consistency of medical products and contrasts the highly controlled GMP facility with a standard research laboratory. Key GMP requirements detailed include the use of classified cleanrooms (e.g., ISO 5-8 air quality), full sterile gowning protocols, rigorous equipment qualification and validation, and the implementation of Standard Operating Procedures (SOPs) for every step to ensure process consistency and end-to-end product traceability.
This article provides a detailed deconstruction of the cell therapy production workflow, using CAR-T therapy as a primary example. It outlines the core manufacturing stages, starting with cell collection and isolation, followed by activation using methods like autologous antigen-presenting cells to trigger cell division. The text then describes genetic modification via lentiviral systems or electroporation, and large-scale expansion in bioreactors. It emphasizes that all key operations must occur in an A-class laminar flow environment within a B-class background to comply with GMP and prevent contamination. The role of quality systems like CMC (Chemistry, Manufacturing, and Controls) is also explained.
This piece offers a holistic analysis of the cell therapy ecosystem, examining both the scientific processes of production and the business dynamics that shape the industry. It provides a model process diagram for centralized manufacturing, detailing the entire workflow from harvesting biological material to release testing and final clinical administration. It also profiles the key industry players, including established pharmaceutical giants (Novartis, Gilead), innovative biotech startups (Bluebird Bio), and the specialized Contract Development & Manufacturing Organizations (CDMOs) like Lonza and Catalent, whose expertise in scaling production and navigating regulatory requirements makes them indispensable partners. The text also enumerates the significant challenges, including biological variability, strict regulatory oversight, and high costs, with facilities often exceeding $100 million in investment.
This article charts the course from industrial manufacturing to market, focusing on the infrastructure and regulatory hurdles involved. It specifies the need for GMP-compliant facilities, particularly clean workshops with B/A aseptic environments to prevent microbial contamination. It also details the specialized equipment required, such as bioreactors for cell expansion and flow cytometers for phenotypic analysis, and the robust quality control (QC) systems needed to ensure product safety and efficacy. The text then analyzes the primary obstacles to commercialization, including high prices that conflict with existing medical insurance systems—where a survey showed only 15% of U.S. commercial insurers fully cover CGT costs—and disparities in global regulatory standards between the FDA, EMA, and NMPA.
The critical role of Contract Development and Manufacturing Organizations (CDMOs) in the CGT field is the focus of this article. It defines CDMOs as partners that offer a full suite of services, including early-stage process development, GMP-compliant manufacturing, analytical method validation, and regulatory submission support. The article highlights that CDMOs are uniquely equipped to handle sensitive biological materials and possess state-of-the-art infrastructure like ISO-class cleanrooms. By outsourcing these complex and capital-intensive operations, therapy developers can accelerate timelines, reduce risk, and leverage deep regulatory expertise for critical submissions like Investigational New Drug (IND) applications and Biologics License Applications (BLAs).
This article provides a deep analysis of the innovative payment solutions required to address the high upfront costs of CGTs, which can range up to $4 million per dose. It details the outcomes-based agreements (OBAs) used in the U.S., where the Centers for Medicare and Medicaid Services (CMS) negotiates with manufacturers to link reimbursement to actual patient treatment results. This model is contrasted with strategies in major European countries, such as risk-sharing agreements and payments in installments based on clinical endpoints, as seen in Germany and Italy. The article also explores practices in the Asian market, where countries like Japan and South Korea are using a combination of medical insurance negotiations and commercial insurance products to improve patient access.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION