On June 10, 2025, the Center for Drug Evaluation (CDE) of the National Medical Products Administration released the "Scope, Classification and Interpretation of Advanced Therapeutic Drugs (Draft for Comment)", announcing that advanced therapeutic drugs in China are classified into three major categories: cell therapy drugs (CTMPs), gene therapy drugs (GTMPs), and others. At present, China's cell therapy industry has entered a new stage of running on par with the international advanced level.
Cell and Gene Therapy (CGT) refers to an emerging treatment method that introduces exogenous genetic material into target cells to modify or manipulate the expression of genes and change the biological characteristics of cells to achieve therapeutic effects. In China, CGT products mainly include human-derived stem cells and their derivative cell therapy products, immune cell therapy products and gene therapy products. Unlike traditional therapies, CGT have shown great potential in the treatment of numerous diseases in humans, such as malignant tumors, rare diseases, genetic disorders and infectious diseases. However, due to the significant individual differences in CGT, it is more novel and complex1.We can get a sense of the great potential of cell and gene therapy through some cases.
The world's first case of treating AIDS and leukemia with gene-edited stem cells. The team led by Deng Hongkui from the Peking University-Tsinghua University Center for Life Sciences has utilized CRISPR/Cas9 technology to conduct gene editing (knocking out the CCR5 gene) on human hematopoietic stem cells. The gene-edited stem cells can stably rebuild the hematopoietic system for a long time in animal models, and the peripheral blood cells generated by them possess the capacity to combat AIDS and leukemia. Successfully treated a patient who was suffering from both AIDS and acute lymphoblastic leukemia. This means that Chinese researchers have for the first time completed the treatment of AIDS and leukemia patients with gene-edited stem cells2. Massachusetts General Hospital treated three patients with recurrent glioblastoma using a new CAR-T therapy (CARv3-TEAM-E), targeting EGFRvIII mutations and enhancing immune attacks. The rapid efficacy of CAR-T in solid tumors (especially brain tumors) was proved for the first time, providing a new option for refractory cancers3. Professor Shu Yilai from the Eye and ENT Hospital of Fudan University led the collaboration to complete the world's first clinical trial on gene therapy for hereditary deafness. This is the world's first successful case of gene therapy for hereditary deafness, bringing hope to patients with auditory neuropathy. One successful treatment case after another has demonstrated the application potential of CGT in curing difficult and complicated diseases, ushering in a new era of CGT4.
CGT was initially mainly applied in the treatment of genetic diseases and has gradually been widely used in malignant tumors, infectious diseases, cardiovascular diseases and autoimmune diseases. In recent years, with the continuous improvement of technology, the safety and effectiveness of gene therapy have been enhanced, and breakthrough achievements have been made in the clinical treatment of various hereditary rare diseases, such as hemophilia, leukodystrophy, Duchenne muscular dystrophy and congenital amaurosis. The specific application scenarios are as follows (Fig.1).
The CGT Manufacturing Conference invites industry experts in the field to share the latest research progress and clinical practice experience. Through in-depth discussions on technical challenges and solutions, it promotes cross-border collaboration and innovation in CGT technology, accelerates its wide application in clinical practice, and provides new hope and progress for global health. The participating groups include enterprises engaged in CGT research and production, clinical doctors, CRO/CDMO enterprises, enterprises of production process equipment and consumables, research institutes and some government review institutions, etc. During the meeting, there will be a brainstorming session among industry leaders, jointly discussing fundamental innovation and market competition, and guiding the direction and strategic layout for the future development of the industry. Researchers can share the latest research achievements in technologies such as gene editing, bringing new ideas and methods to the manufacturing of CGT products. The introduction of practical experience in the product manufacturing process by enterprise representatives can promote the optimization of production techniques. In addition, the conference also provided opportunities for cooperation within the industry. Enterprises can explore the possibility of jointly developing new products and sharing technology platforms. Research institutions and enterprises can also cooperate in aspects such as technology transfer and clinical trials, jointly promoting the development of the CGT industry.
The manufacturing of CGT is a complex and meticulous process. The manufacturing process of CGT is a deep exploration of life science. Every link contains complex technical principles and meticulous operational requirements. Its complexity is not only reflected in the technical aspect, but also in the strict requirements for quality control and compliance. From the collection, separation and culture of cells, to the editing of genes and the construction of vectors, and then to the purification and quality inspection of the final product, every step is like being meticulously crafted in a microscopic world. The slightest mistake may affect the safety and effectiveness of the entire therapeutic product.
With more and more CGT being developed and obtaining more and more regulatory approvals, the demand for industrialization is emerging and is imminent. The manufacturing and commercialization of CGT are expected to achieve significant breakthroughs and developments in the next 50 years. At present, the upstream of cell and gene therapy in China mainly consists of biological science tools and cell and gene therapy CRO/CDMO (Fig.2). Midstream cell and gene therapy mainly consists of new drug research and development companies for cell and gene therapy. The downstream is terminal sales, including hospitals, retail pharmacies, etc.
The UK has established a CGT center, which facilitates the advancement throughout the entire process of CGT products from research and development to clinical application by guiding enterprises in their research and development, providing financial support, promoting interaction between enterprises and regulatory authorities, building advanced facilities for enterprises to develop production processes, and establishing a skills training platform to enhance the cultivation of industry talents. At present, China is actively promoting the construction of scientific research and transformation platforms in key innovation fields such as CGT. It is suggested that the subsequent construction and operation of scientific research and transformation platforms in China can consider adopting a joint management model of "government + market", with a focus on infrastructure construction, talent cultivation, development of common technical problems, and exploration of payment methods, etc. To promote CGT products to move from research and development to clinical application as soon as possible. As a cutting-edge field in global biomedicine, CGT is gradually becoming a key direction for the future development of biomedicine and has shown great application potential in many areas where traditional treatments are ineffective for serious or refractory diseases.
With the continuous advancement and innovation of technology, the manufacturing processes of cell and gene therapy will be constantly improved and optimized, bringing hope and the possibility of cure to more patients.
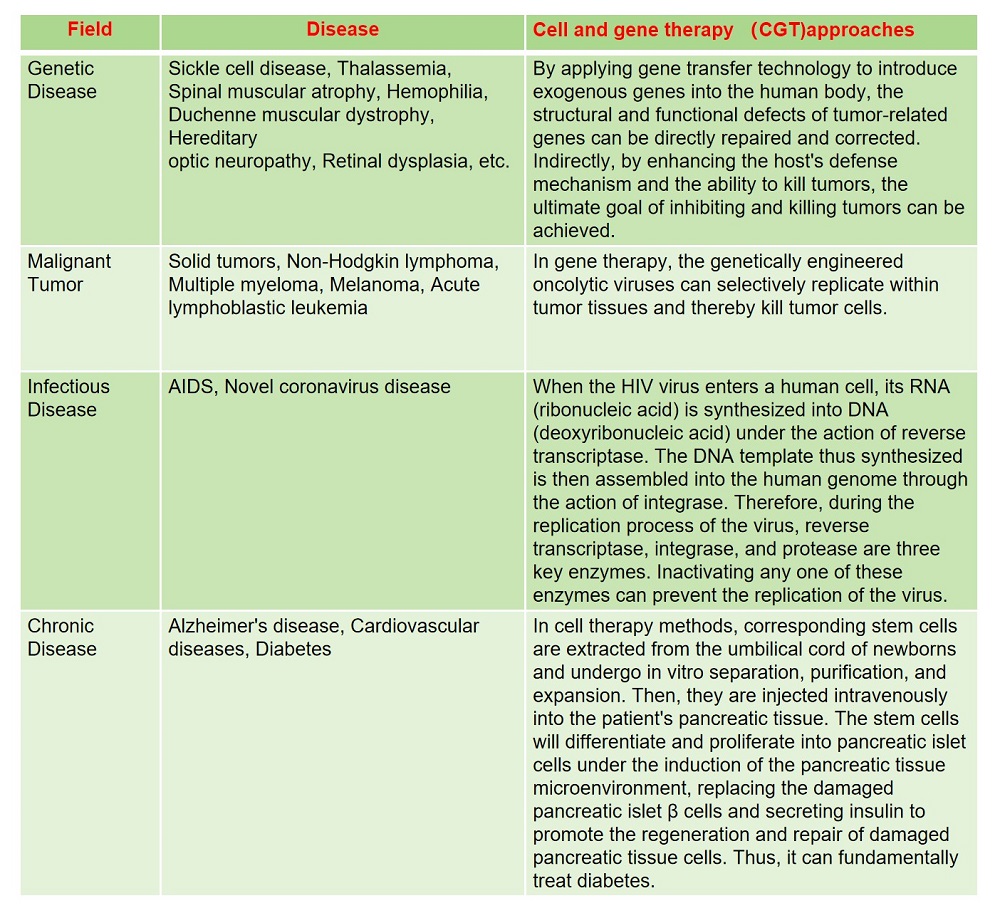 Fig.1 The distribution of application scenarios in the CGT industry.
Fig.1 The distribution of application scenarios in the CGT industry.
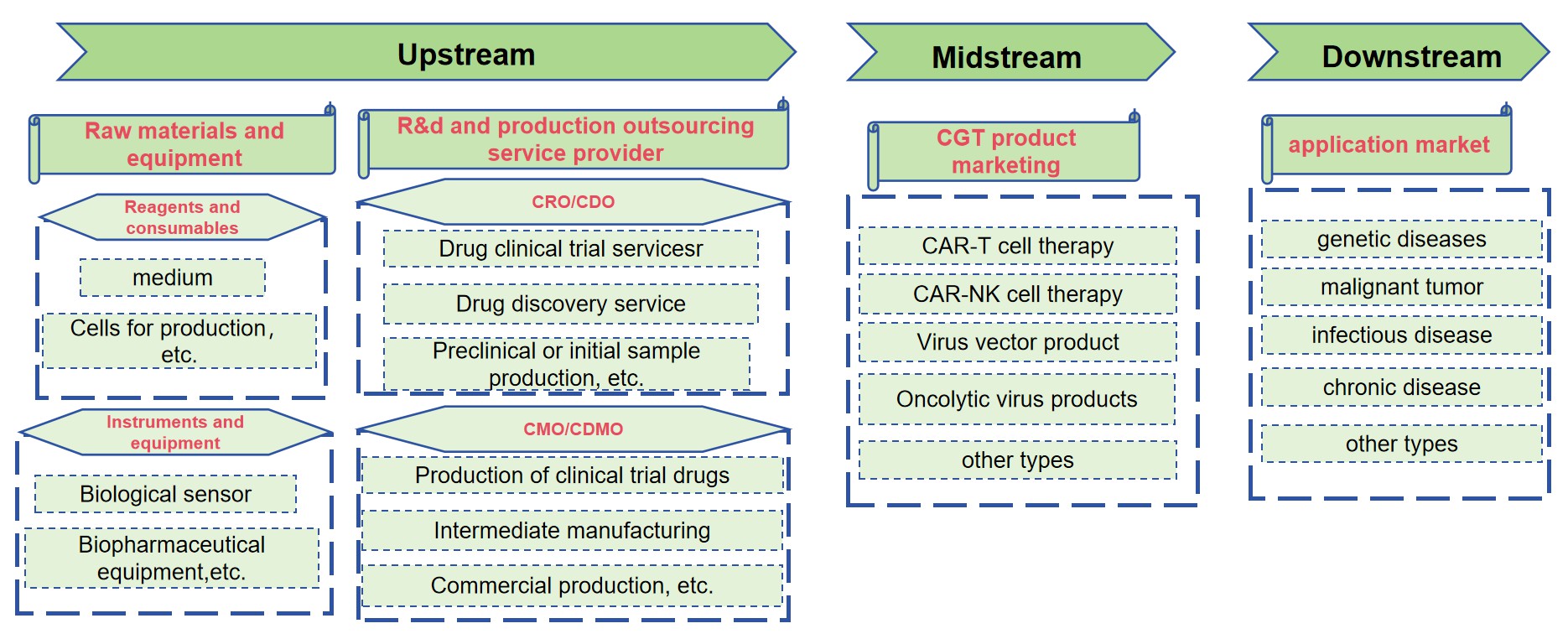 Fig.2 The industrial chain structure of CGT.
Fig.2 The industrial chain structure of CGT.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION