Cell therapy represents one of the most revolutionary advances in modern medicine. Unlike traditional drugs that merely treat symptoms, cell therapies aim to repair, replace, or regenerate damaged tissues or immune functions by harnessing the power of living cells.
Cell therapy represents a revolutionary frontier in medicine, offering not just treatment but potential cures by harnessing the body's own cellular repair mechanisms. This groundbreaking approach has already shown remarkable success in oncology, where CAR-T cell therapies have achieved complete remission in patients with otherwise untreatable blood cancers. Beyond cancer, these living medicines are demonstrating transformative potential across multiple therapeutic areas - regenerating damaged heart tissue in heart failure, restoring vision in degenerative eye diseases, and even repairing spinal cord injuries. As research advances, the scope continues to expand to include common chronic conditions like diabetes, Parkinson's, and autoimmune disorders. What makes cell therapy truly paradigm-shifting is its potential to move medicine beyond symptom management to actual biological restoration, promising not just extended life but restored quality of life. The rapid progress in this field suggests we may be on the cusp of a new era where many currently incurable diseases become treatable, if not curable, through cellular approaches. This scientific revolution is reshaping our fundamental understanding of disease treatment and prevention.In essence, cell therapy doesn't just treat-it transforms.
Cell therapy manufacturers play a critical role in translating innovative biomedical science into tangible, life-changing treatments. In modern medicine, their function goes far beyond simple production; they serve as the essential bridge between laboratory discovery and clinical application. As highlighted in the literature, the journey from a patient-specific cell sample to a viable, regulatory-compliant therapy requires mastery over a complex series of operations-from cell sourcing, isolation, and genetic modification, to large-scale expansion, cryopreservation, and quality control testing.
Manufacturers must design processes that align with Good Manufacturing Practices (GMP), ensuring each product is consistent, sterile, and safe despite inherent variability in patient-derived cells. For autologous therapies in particular, where each treatment is customized to the patient, this challenge is magnified. As such, manufacturing protocols must be flexible yet standardized, supported by validated assays that confirm cell identity, purity, potency, and functionality.
Moreover, successful commercialization of these therapies relies heavily on manufacturers' ability to scale production—either by expanding capacity (scale-out) or increasing batch sizes (scale-up)—without compromising quality. Contract Manufacturing Organizations (CMOs) are often enlisted for this purpose, and their expertise in technology transfer, regulatory compliance, and bioprocess optimization is crucial to product success.
Ultimately, cell therapy manufacturers are not just technicians; they are architects of therapeutic reliability. Their precision and infrastructure form the backbone of regenerative medicine and personalized cellular treatments. In a field where a single product failure could jeopardize patient safety or regulatory approval, the role of manufacturers is both central and indispensable to the future of modern medical care.
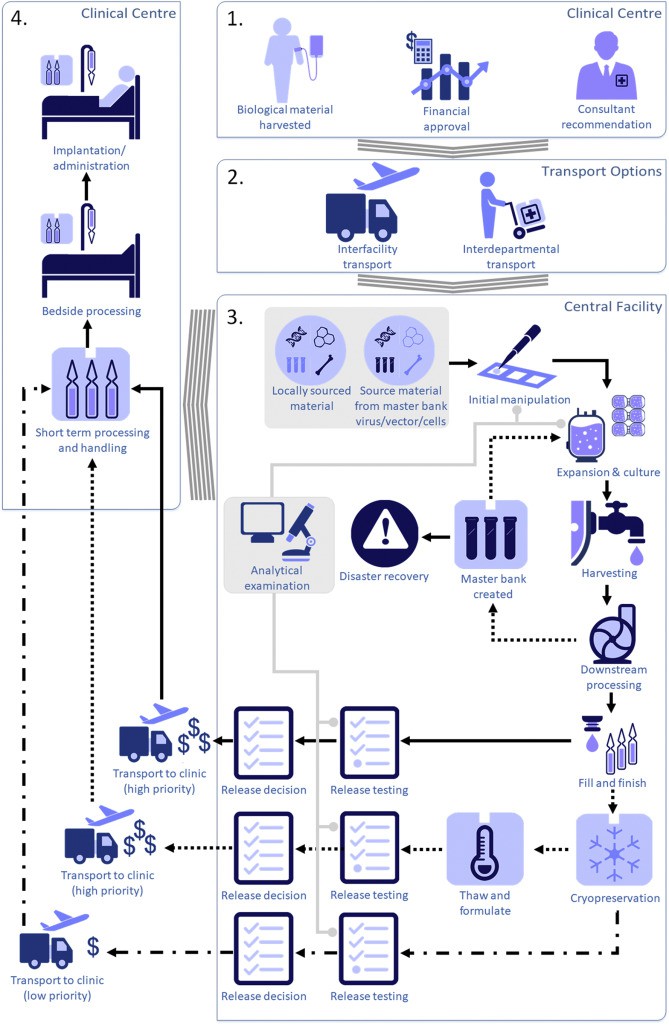 Fig.1 Model process diagram for centralised manufacturing of cell and gene therapy products1.
Fig.1 Model process diagram for centralised manufacturing of cell and gene therapy products1.
The cell therapy manufacturing sector is a dynamic and rapidly evolving ecosystem, shaped by the interplay of diverse players, economic pressures, and logistical complexities.
Established pharmaceutical giants like Novartis, Gilead/Kite Pharma, and Bristol Myers Squibb have entered the cell therapy space through acquisitions, partnerships, and in-house development. Their deep pockets and global infrastructure enable large-scale production and commercialization of therapies like CAR-T cells.
Innovative startups often drive early-stage research and development, focusing on niche applications or novel technologies. These companies, such as Bluebird Bio and Adaptimmune, frequently collaborate with larger manufacturers or CDMOs to scale up production.
Specialized CDMOs like Lonza, Catalent, and Thermo Fisher Scientific provide essential manufacturing services to smaller biotechs and even large pharma companies. Their expertise in scaling up production and navigating regulatory requirements makes them indispensable partners in the cell therapy supply chain.
Despite the tremendous potential of cell therapies, manufacturers face a complex set of challenges that distinguish this field from traditional pharmaceutical production. One of the most significant hurdles is biological variability. Unlike chemical drugs that are uniform and reproducible, cell therapies start with cells taken directly from patients. These cells can vary greatly in quality, viability, and function based on the individual's age, health status, and genetics. As a result, every manufacturing batch is inherently different, demanding highly adaptable and precise production protocols.
Compounding this variability is the strict regulatory oversight imposed by agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Cell therapy products must adhere to rigorous standards of identity, purity, potency, and sterility. Manufacturers must operate under Good Manufacturing Practice (GMP) conditions and establish robust quality control measures at every stage—from cell isolation and expansion to final product testing and release. Navigating these regulatory pathways can be particularly demanding for early-stage biotech companies and academic developers.
Table 1 cell and gene-based therapies (CGTs) receiving EMEA marketing authorization
| Product | Mechanism of action | Status |
| Glybera® | A gene therapy product repairing faulty fat metabolism | Authorized |
| ChondroCelect® | An autologous cartilage cell therapy which expands patient biopsies | Withdrawn |
| Provenge® | Treatment for metastatic castration-resistant prostate cancer | Withdrawn |
| Holoclar® | Treatment for burn induced limbal stem cell deficiency | Conditional |
| MACI® | A cultured chondrocyte product for cartilage defects | Suspended |
| Imlygic® | A viral treatment for recurrent melanoma | |
| Strimvelis® | A gene therapy for a type of severe combined immunodeficiency | Authorized |
| Zalmoxis® | An adjunctive T-cell therapy for use with stem cell transplants | Conditional |
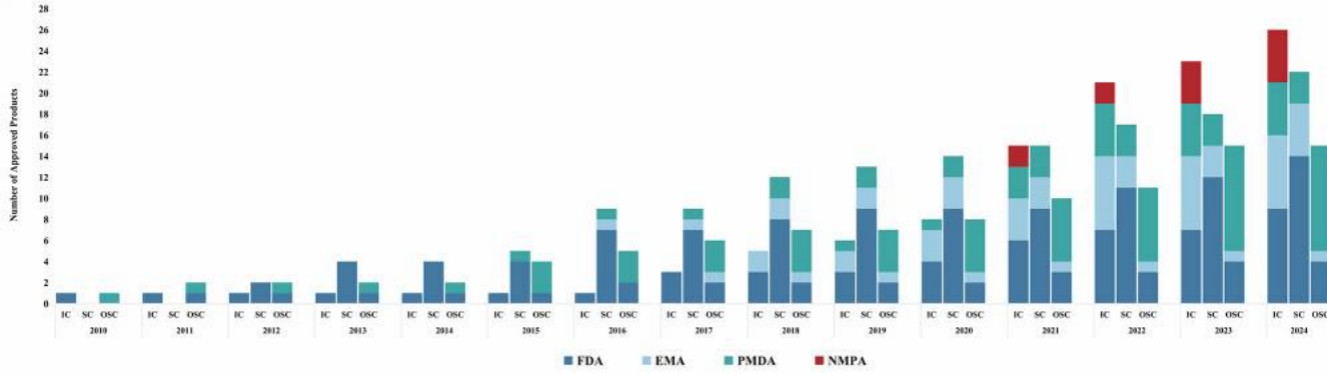 Fig.2 Cumulative Number of Cell Therapy Products Approved for Marketing by the NMPA, FDA, EMA and PMDA (2010-2024). Abbreviations: FDA, Food and Drug Administration; EMA, European Medicines Agency; PMDA, Pharmaceuticals and Medical Devices Agency; NMPA, National Medical Products Administration. IC, Immune Cell; SC, Stem Cell; OSC, Other Somatic Cell. Note: The cut-off date was August 1, 20242
Fig.2 Cumulative Number of Cell Therapy Products Approved for Marketing by the NMPA, FDA, EMA and PMDA (2010-2024). Abbreviations: FDA, Food and Drug Administration; EMA, European Medicines Agency; PMDA, Pharmaceuticals and Medical Devices Agency; NMPA, National Medical Products Administration. IC, Immune Cell; SC, Stem Cell; OSC, Other Somatic Cell. Note: The cut-off date was August 1, 20242
The cell therapy manufacturing landscape is undergoing a technological revolution. AI-driven process optimization is enabling real-time monitoring and adaptive control of critical parameters, significantly improving yield and consistency while reducing batch failures. 3D bioprinting is emerging as a disruptive technology, allowing for precise spatial organization of cells and biomaterials to create more physiologically relevant therapeutic products. The adoption of modular manufacturing systems offers flexible, scalable solutions that can be rapidly deployed to meet fluctuating production demands.
In the realm of allogeneic therapies, breakthroughs in gene editing technologies (e.g., base editing, prime editing) are enabling the development of "universal donor cells" with enhanced immune evasion properties. The emergence of induced pluripotent stem cell (iPSC) banks is creating standardized starting materials that could dramatically improve manufacturing consistency while reducing costs. These technological leaps are complemented by advanced analytical technologies such as single-cell sequencing and live-cell imaging that provide unprecedented quality control capabilities.
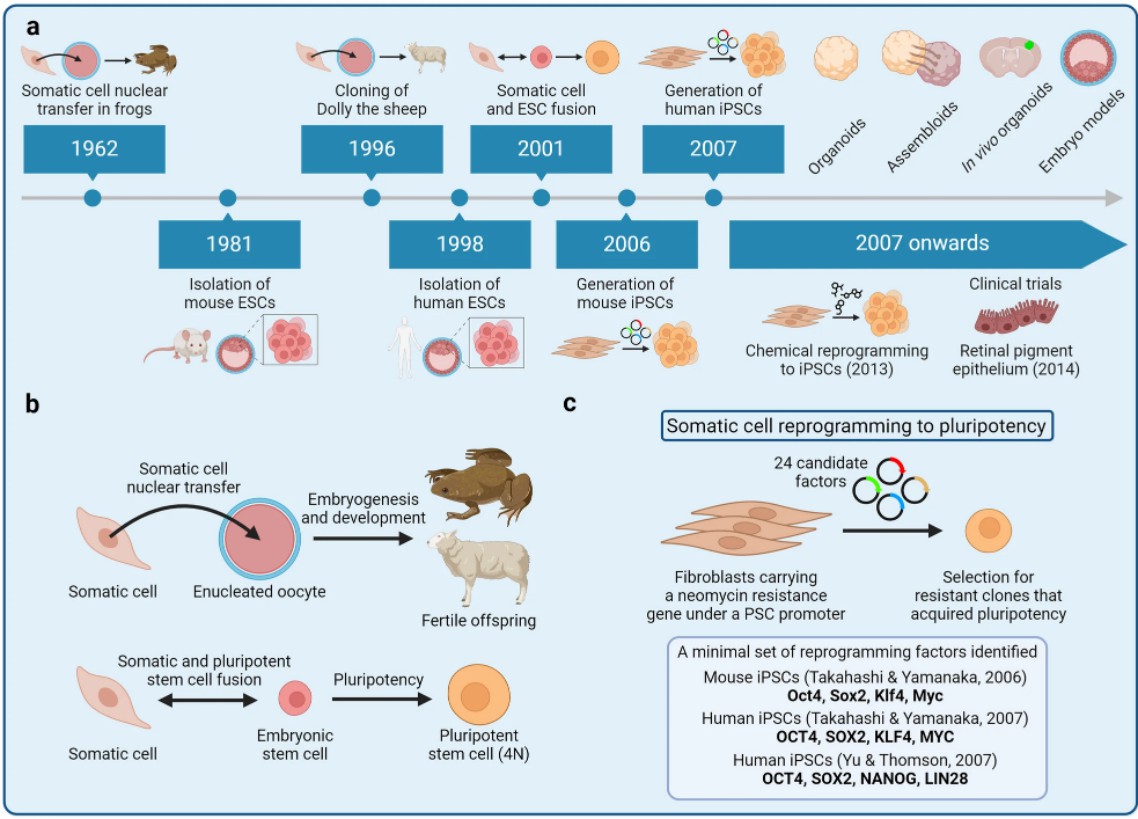 Fig. 3 Development of the iPSC technology3.
Fig. 3 Development of the iPSC technology3.
The cell therapy manufacturing sector stands at a critical juncture, where overcoming current limitations will determine whether these revolutionary treatments can achieve their full potential. Three fundamental challenges must be addressed:
Current manufacturing costs remain prohibitively high ($300,000-$500,000 per treatment)
Complex supply chains create bottlenecks in global distribution
Specialized facilities require massive capital investments (often exceeding $100 million)
Scalability limitations in autologous therapies (patient-specific production)
Variability in cell product quality between batches
Evolving regulatory requirements across different regions (FDA vs. EMA vs. other markets)
Reimbursement models struggling to accommodate high upfront costs
Limited manufacturing capacity creating treatment delays
Complex logistics for cell collection, transport and delivery
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION