The research and development of cell therapy for diabetes is a long and tortuous process, which embodies the efforts of countless researchers. Its origin can be traced back to the in-depth study of cell biology and the pathogenesis of diabetes.Early research mainly focused on animal experiments. Researchers tried to transplant cells from different sources into diabetic animal models and observe their effects on blood sugar levels. In animal experiments, the use of embryonic stem cells (ES cells) as starting materials shows certain potential. ES cells have strong self-renewal ability and multi-directional differentiation potential, and can theoretically differentiate into islet β cells. Through a series of precise cell culture steps, scientists successfully induced ES cells to differentiate into pancreatic progenitor cells, and then developed into islet cell clusters1.
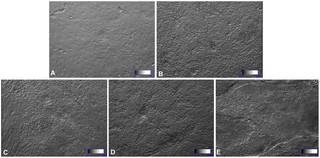 Fig.1 Differentiation of hESCs into a pancreatic lineage in a four-stage protocol1.
Fig.1 Differentiation of hESCs into a pancreatic lineage in a four-stage protocol1.
(A) hESC colonies were passaged onto 1% Matrigel-coated dishes for attachment. (B) In stage 1, the hESC cluster was incubated in RPMI1640 with 0.2% FBS, 0.5×N2, 0.5×B27, 100 ng/mL activin A, and 1 µM wortmannin. (C) In stage 2, cells were culturing in RPMI1640 with 0.5% FBS, 0.5% ITS, 0.5×B27, 2 µM RA, 20 ng/mL FGF7, and 50 ng/mL Noggin. (D) In stage 3, cells were cultured in high-glucose DMEM supplemented with 0.5% FBS, 1% ITS, 1×N2, and 50 ng/mL EGF. (E) In stage 4, the cells were cultured in DMEM/F12 with 1% ITS, 10 ng/mL bFGF, 10 mM nicotinamide, 50 ng/mL exendin-4, and 10 ng/mL BMP4 for maturation. Scale bars: 100 µm.
With the initial success of animal experiments, researchers began to move cautiously towards human experiments. In the early stage of human trials, there are many challenges, including the safety of cell source, immune rejection and the uncertainty of treatment effect. Initially, some clinical trials used islet allografts, that is, islet cells from donors were transplanted into patients. Although the blood sugar control of some patients has been significantly improved after transplantation, the donor islet cells are limited in source and the problem of immune rejection is serious.
In order to solve these problems, researchers continue to explore new cell sources and treatment strategies. The emergence of induced pluripotent stem cells (iPS cells) technology has brought a new dawn for diabetic cell therapy. IPS cells are reprogrammed to restore adult cells to pluripotent states similar to embryonic stem cells. Compared with ES cells, iPS cells avoid the ethical controversy caused by the use of embryonic stem cells, and can be obtained from patients' own cells in theory, which greatly reduces the risk of immune rejection2. Related research shows that somatic cells such as skin fibroblasts can be reprogrammed into iPS cells by specific transcription factor combinations, and then directionally induced to differentiate into islet β cells.
In the process of human trials, researchers continue to optimize the treatment plan. From simple cell transplantation at first to advanced cell delivery technology and immune regulation strategy to improve the survival rate and functional durability of transplanted cells.
In the cell therapy of type 1 diabetes mellitus, the choice of stem cell source is very important, and stem cells from different sources have their own unique characteristics and application potential.
1. Embryonic stem cells (ES cells):ES cells, derived from early embryos, have the totipotency of infinite proliferation and multi-directional differentiation, and can differentiate into various tissue cells of human body, including islet β cells. Through well-designed cell culture scheme, ES cells can be induced to gradually differentiate into pancreatic progenitor cells along the development path of pancreas, and finally form functional islet β cells.
2. Induced pluripotent stem cells (iPS cells): IPS cells are similar to ES cells in function, and can also differentiate into islet β cells for diabetes treatment. The advantage of iPS cells is that they avoid the ethical problems brought by embryonic stem cells, and can theoretically be obtained from patients' own cells to achieve personalized treatment, which greatly reduces the possibility of immune rejection.
Directional differentiation of stem cells into mature islet β cells is the core link of diabetic cell therapy, but the differentiation efficiency of this process still needs to be improved. In the laboratory, by simulating the microenvironment of pancreatic development in vivo and adding various growth factors, cytokines and signal pathway regulators, researchers induced stem cells to gradually differentiate into islet β cells.
In order to improve the efficiency of differentiation, researchers have adopted various strategies. On the one hand, the molecular mechanism of pancreatic development is deeply studied, and more key regulatory factors and signal pathways are excavated to optimize the differentiation scheme; On the other hand, advanced Qualcomm screening technology was used to screen a large number of compounds and biological materials, looking for new inducers that can promote the efficient differentiation of stem cells into islet β cells3.
Successful transplantation of differentiated islet β cells into patients and ensuring their long-term survival and normal function are the keys to the clinical application of diabetic cell therapy. At present, the commonly used transplantation technologies mainly include the following:
Portal vein transplantation: this is a more traditional transplantation method, in which islet cells are injected into the liver through portal vein. Liver has abundant blood supply and suitable microenvironment, which is beneficial to the colonization and survival of islet cells. Islet cells can interact with hepatic sinusoidal endothelial cells in the liver to obtain nutrients and oxygen, and at the same time secrete insulin into the blood circulation to regulate blood sugar levels.
Subcutaneous transplantation: Subcutaneous tissue has the advantages of simple operation, easy observation and monitoring, and has become one of the hot transplant sites in recent years. In order to improve the survival rate and function of subcutaneous transplanted cells, researchers have developed a variety of cell delivery systems based on biomaterials, such as microcapsules and hydrogels4.
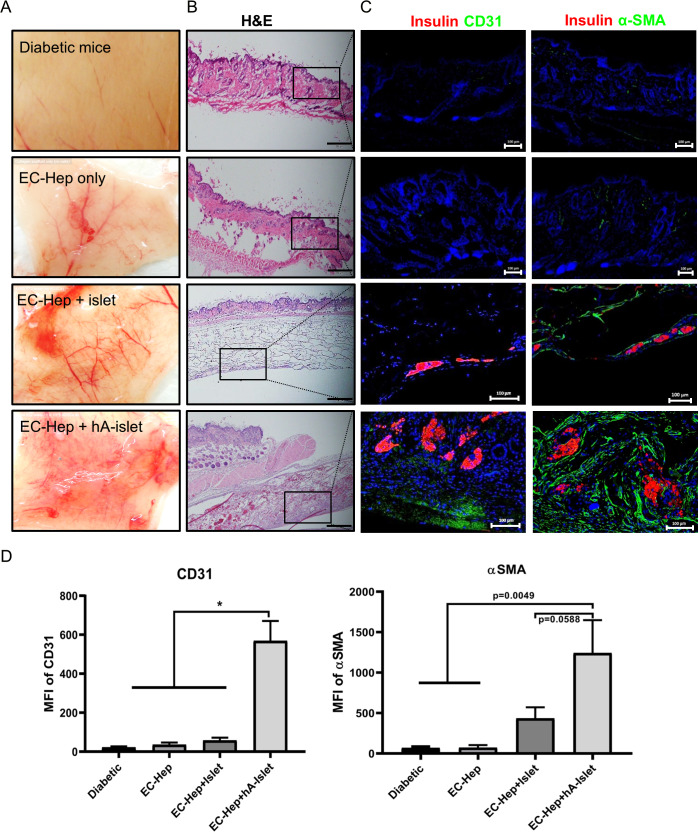 Fig.2 Vascularization effect of subcutaneous transplantation system4.
Fig.2 Vascularization effect of subcutaneous transplantation system4.
(A) Gross views of the grafted site 7 days after transplantation. (B) H&E of grafted site. (C) The degree of vascularization was evaluated according to the number of vessels for anti-CD31 and alpha smooth muscle actin (αSMA) immunostaining. Immunohistochemistry on graft tissues stained with anti-mouse CD31 antibody by the immunofluorescence methods. CD31 and αSMA staining is shown in green. Nuclei were stained with 4', 6-diamidino-2-phenylindole (DAPI), blue). Negative controls were mice with transplantation of cell-free esterified collagen-heparin (EC-Hep). (D) CD31 and αSMA positively stained cells counted in five different fields were imaged by confocal microscopy and the mean fluorescence intensity was calculated using ImageJ software. Results were analyzed by analysis of variance and the graphs represent mean±SD (*p<0.01). The intensity (CD31+ and αSMA+) in the heparin-esterified collagen-human adipose-derived stem cells (hADSC) (HCA)-islet sheet were significantly higher than in the other groups (scale bars denote 100 µm). Immunostained samples of representative sections from experimental groups and mice with diabetes are shown. MFI, mean fluorescence intensity.
In order to improve the survival rate and functional durability of transplanted cells, it is necessary to combine immune regulation strategies.
The enlargement from small-scale preparation in the laboratory to large-scale industrial production is a major obstacle in the clinical transformation of cell therapy for diabetes. In the laboratory environment, researchers can accurately control every step, from cell inoculation to differentiation, to ensure the quality and activity of cells. However, large-scale production will introduce complex variables and destroy this accuracy.
Stability of cell expansion: As the starting material, stem cells need large-scale expansion to produce enough functional islet β cells for clinical use. But in the process of amplification, the growth rate, differentiation potential and genetic stability of cells are highly sensitive to environmental factors. Small fluctuations in medium composition, temperature or pH value may lead to abnormal cell proliferation or deviation from the target differentiation direction. Maintaining the uniformity and stable function of cells under large-scale culture conditions is still the core challenge, which requires optimized bioreactor system and real-time monitoring of cell state.
Large-scale production inevitably involves high costs, covering raw materials, equipment and personnel. The key inputs such as special culture medium, growth factors and biomaterials needed for cell delivery system are expensive, and the cost increases exponentially with the expansion of production scale. In addition, strict quality inspection requires advanced analytical equipment and professional technicians, which further pushes up the cost. In order to solve this problem, this field is exploring various strategies: developing low-cost serum-free medium; Transforming existing biological processing equipment to reduce capital investment; Reduce labor costs through automation technology. These efforts are aimed at making cell therapy economically feasible without sacrificing safety and effectiveness.
Stem cell therapy relies on a variety of special raw materials, including pluripotent cell lines, cytokines and biodegradable scaffolds. Many of these materials are fragile and need strict storage conditions, such as low temperature storage and aseptic environment. Establishing a stable supply chain is very important to avoid production interruption. This includes working with qualified suppliers to standardize the quality of materials; Implement multi-source procurement redundancy to alleviate the shortage risk; Develop a low-temperature preservation scheme to extend the shelf life of materials. Traceability system is also indispensable, which can completely record the source and processing of materials to meet regulatory requirements.
In the process of diabetes cell therapy development, the application of embryonic stem cells is at the heart of a strong ethical controversy. Because these cells are usually derived from the blastocyst stage of early embryos, their acquisition usually involves the destruction of the embryo, which is ethically controversial about "killing life"5. To address these ethical disputes, researchers and policymakers have explored multiple solutions. For one, they are actively pursuing alternatives—like the aforementioned induced pluripotent stem cell (iPS cell) technology, which reprograms adult cells to a pluripotent state, thus sidestepping the ethical concerns tied to embryonic stem cell use.
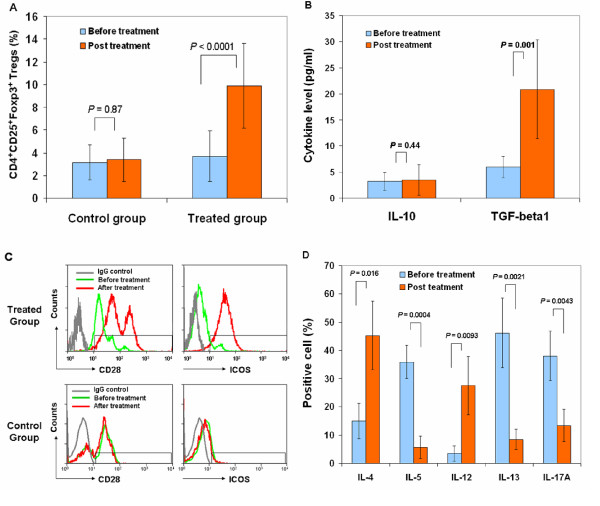 Fig.3 Effect of stem cell education therapy on regulatory T cells5.
Fig.3 Effect of stem cell education therapy on regulatory T cells5.
Patient lymphocytes were isolated from peripheral blood by Ficoll-Hypaque (γ = 1.077) for flow cytometry analyses in T1D patients at baseline and 4 weeks after Stem Cell Educator therapy. Isotype-matched IgG served as control. (A) Flow Analysis of CD4+CD25+Foxp3+ Tregs demonstrating an increase in the percentage of Tregs at 4 weeks post-treatment. (B) Cytokine ELISAs demonstrating an increase in TGF-β1 but not IL-10 at 4 weeks post treatment. (C) Flow cytometry on co-stimulating molecules indicating increases in CD28 and ICOS at 4 weeks post-treatment with Stem Cell Educator therapy (top panels). Control group failed to show increases (bottom panels). (D) Flow analysis of intra-cellular cytokines demonstrating differential effects on key interleukins at 4 weeks post-treatment. Data are representative of preparations from all T1D participants (n = 12) that received Stem Cell Educator therapy.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION