In recent years, medicine has entered a new era—one where living cells are used not just to support the body, but to actively treat disease. From reprogrammed immune cells that fight cancer to stem cells that help regenerate damaged tissue, cell therapy is offering new hope for patients with conditions once thought incurable1.
But making medicine from living cells is nothing like making pills or vaccines. These therapies must be carefully grown, tested, and handled under strict conditions to ensure they are both safe and effective. This is where Good Manufacturing Practice (GMP) comes in.
In this article, we explore how cell therapy is produced-step by step-from cell collection to delivery to the patient, and why GMP standards are essential in turning scientific breakthroughs into reliable, life-saving treatments.
Specialized technical knowledge in process and GMP facilities are essential to bring these therapies into clinic.
GMP cell therapy refers to cell-based treatments that are manufactured under GMP standards—a strict set of guidelines that ensure the safety, quality, and consistency of medical products. When it comes to therapies made from living cells, maintaining high standards is even more critical, because cells are sensitive, complex, and can easily be compromised by contamination or mishandling.
In GMP cell therapy production, scientists follow detailed protocols to isolate, engineer, expand, and test cells in a way that meets regulatory requirements. Before the cells can be given to a patient, they must pass multiple quality control checks—for identity, purity, potency, and safety. This helps ensure that each dose will perform as expected and won't cause harm.
Without GMP standards, cell therapy would be too risky for clinical use. GMP doesn't just represent good practice—it's what makes cell therapy a safe, reliable, and scalable medical reality. It's the bridge between experimental science and real-world treatment2.
Producing cell therapies isn't like doing science in a university lab. It requires pharmaceutical-grade conditions, where safety, sterility, and consistency are strictly maintained. That's where GMP standards come in.Unlike research labs, which are designed for flexibility and exploration, GMP facilities are built for precision and control. Every aspect of the environment and workflow—from air quality to operator training, from equipment validation to record keeping-is regulated and standardized. This ensures that each batch of cell therapy is safe, reproducible, and traceable.
Here's a clear comparison between a standard lab and a GMP manufacturing facility:
| Aspect | Standard Lab | GMP Cell Therapy Facility |
| Cleanliness Level | Basic lab hygiene | Controlled cleanrooms with classified air quality (ISO 5-8) |
| Contamination Control | Gloves and lab coats | Full sterile gowning; strict entry protocols |
| Documentation | Lab notebooks, variable | Full batch records, electronic logs, deviation tracking |
| Process Consistency | Flexible, trial-based | Standard Operating Procedures (SOPs) for every step |
| Personnel Requirements | General scientific training | GMP-specific training, certification, and requalification |
| Equipment Validation | Basic calibration | Full equipment qualification and maintenance logs |
| Traceability | Limited | End-to-end traceability of cells, reagents, and conditions |
| Quality Control Testing | Not always required | Mandatory in-process and final product testing |
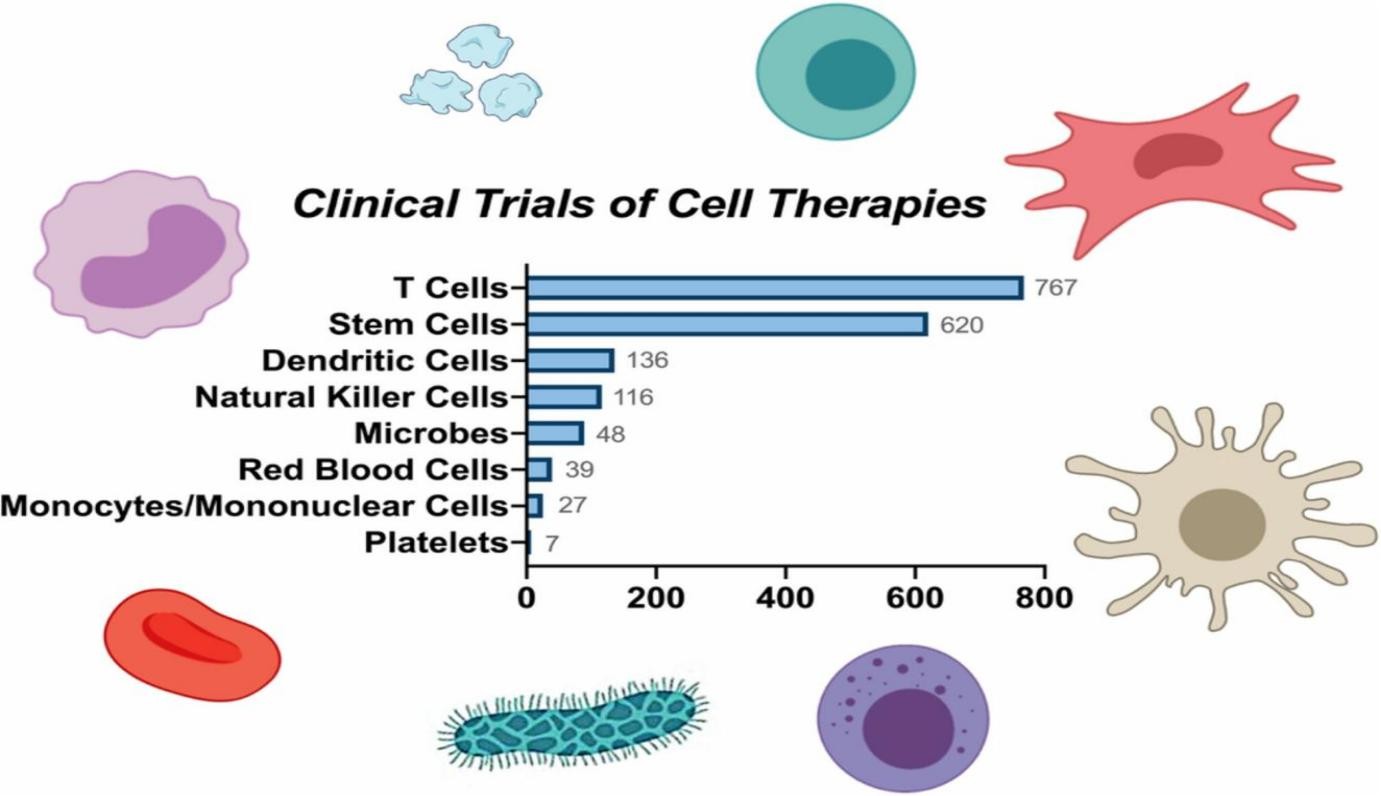 Fig.1 Various types of cell therapies in clinical trials. T cells dominate the current clinical studies of cell therapies, followed by stem cells, dendritic cells, natural killer cells, microbes, red blood cells, mononuclear cells, and platelets3.
Fig.1 Various types of cell therapies in clinical trials. T cells dominate the current clinical studies of cell therapies, followed by stem cells, dendritic cells, natural killer cells, microbes, red blood cells, mononuclear cells, and platelets3.
Producing a cell therapy is not just about science—it's about precision, safety, and transforming living cells into life-saving treatments. This journey, from patient or donor to clinic, involves multiple tightly controlled steps. Here's how cells become medicine.
The process starts by sourcing cells either from the patient (autologous) or a donor (allogeneic), usually via apheresis—a method where blood is filtered to extract specific cells like T cells, with the rest returned. Bone marrow aspiration is also used for stem cells in some cases.
The process begins by collecting the right type of cells. In autologous cell therapy, cells are taken directly from the patient, making the treatment fully personalized and reducing the risk of immune rejection. In allogeneic therapy, cells come from a healthy donor and can be used to treat multiple patients, offering scalability and speed. Once sourced, the desired cells—such as T cells or stem cells—are isolated from blood or bone marrow using techniques like apheresis or aspiration. This is the starting material for all downstream steps.
For certain therapies, especially in cancer treatment, cells are genetically modified to gain new functions. In CAR-T cell therapy, for example, T cells are engineered to express receptors that recognize and attack tumor cells4. This is often done using viral vectors that insert the genetic material into the cells, or CRISPR gene-editing tools for more precise changes. These techniques allow scientists to "program" cells with therapeutic instructions, turning them into powerful tools for targeting disease.
After modification, the engineered cells need to be multiplied into large numbers to achieve therapeutic doses. This expansion happens in bioreactors—specially designed systems that support optimal temperature, nutrients, and oxygen levels. However, scaling up is not as simple as growing more cells; it requires careful monitoring to maintain cell viability, function, and genetic stability. Any variation in this phase can affect the therapy's effectiveness or safety.
Before any therapy reaches a patient, it undergoes rigorous quality control. This includes tests for cell identity, purity, potency, and contamination. Each batch must meet strict regulatory standards, often guided by GMP. Safety testing ensures there are no harmful mutations or infectious agents, while efficacy tests confirm the cells are performing as intended—whether that is killing cancer cells or repairing tissue.
Once cleared for use, the final product is often cryopreserved—frozen at extremely low temperatures to preserve its viability during transport. Specialized logistics are required to ensure these living therapies remain stable from the manufacturing facility to the clinic. When ready, the cells are thawed and infused back into the patient, often through a simple IV. Despite the technical complexity behind the scenes, this moment marks the return of modified, empowered cells—ready to do their job inside the body.
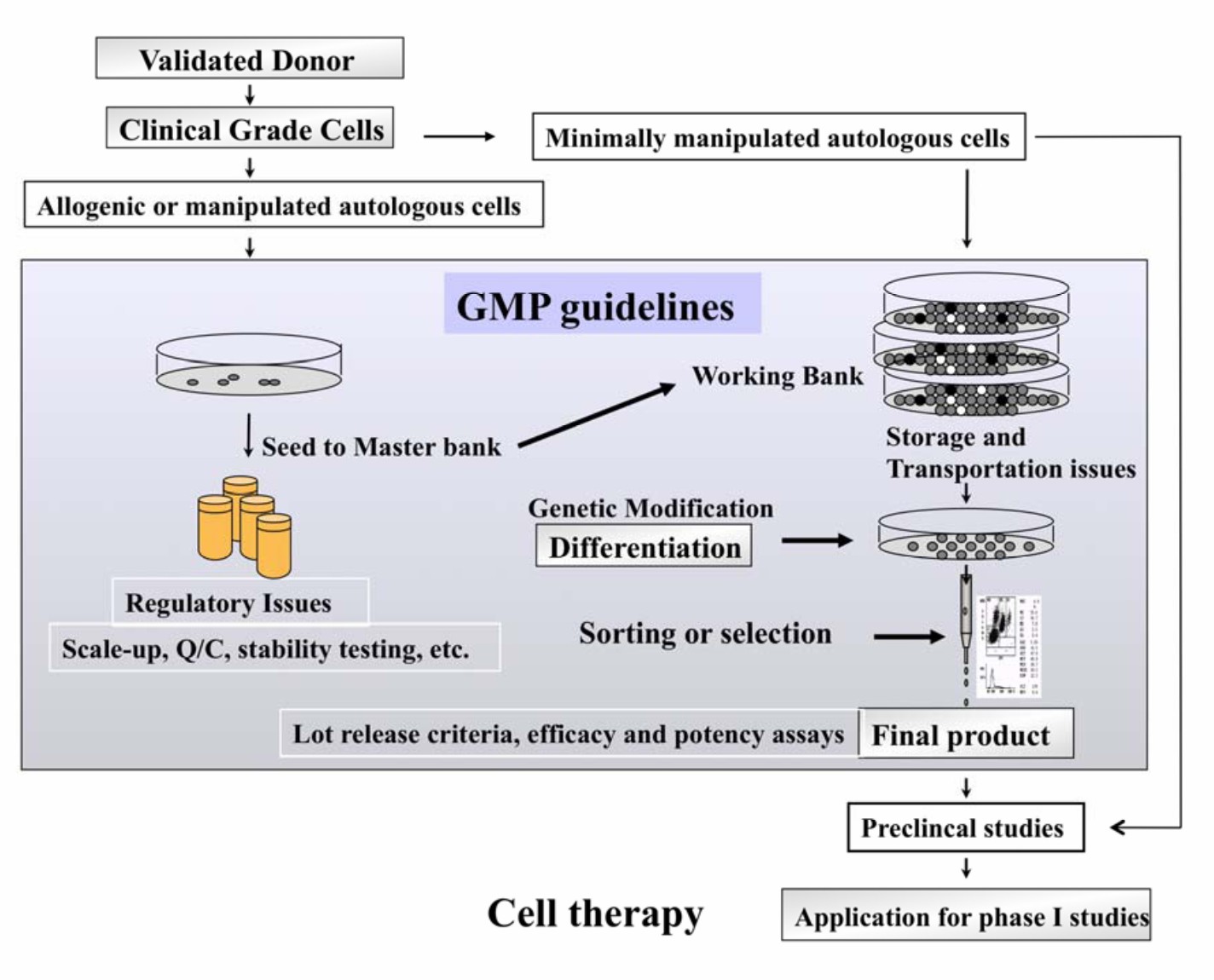 Fig.2 Isolation and Production of Cells Suitable for Human Therapy: Challenges Ahead5.
Fig.2 Isolation and Production of Cells Suitable for Human Therapy: Challenges Ahead5.
Cell therapy promises to revolutionize medicine, but turning a living cell into a safe, consistent (Fig.3), and therapeutic product is no small feat—especially under Good Manufacturing Practice (GMP) conditions. These challenges are particularly acute in personalized therapies, where each product is made for one patient only. Here are the key hurdles:
Unlike pills or vaccines, cell therapies consist of living cells, which can't be sterilized at the end of production. Sterility must be maintained throughout, requiring ultra-clean environments and aseptic techniques from the start. Also, biological variability—such as differences in a patient's tissue—can lead to fluctuations in cell growth and therapy potency.
GMP environments require strict infrastructure: dedicated clean rooms, isolators, air handling systems, and both open and closed systems. The design must ensure minimal cross-contamination risk, especially for multi-product facilities. The cost of building and maintaining these spaces is high, and their complexity rivals that of large pharmaceutical companies.
Highly trained personnel with experience in both GMP standards and complex cell processing are scarce. Most protocols are new, requiring in-depth training. Hospitals must coordinate across departments—from legal to logistics—to ensure compliance and smooth operations.
Constructing even a small GMP facility can cost millions, with ongoing expenses from equipment qualification, maintenance, and quality documentation. Estimating budgets is tricky and underfunding can jeopardize the entire project.
Translating a lab idea into a GMP-compliant product involves redefining procedures, adapting equipment, and ensuring that all reagents meet clinical-grade requirements. Hospitals must align with national and international regulatory agencies, which may differ in their rules—adding another layer of complexity.
Every batch is unique—yet GMP demands standardization. Hospitals must develop flexible, but traceable, protocols to balance the patient-specific nature of cell therapy with regulatory requirements for repeatability and safety.
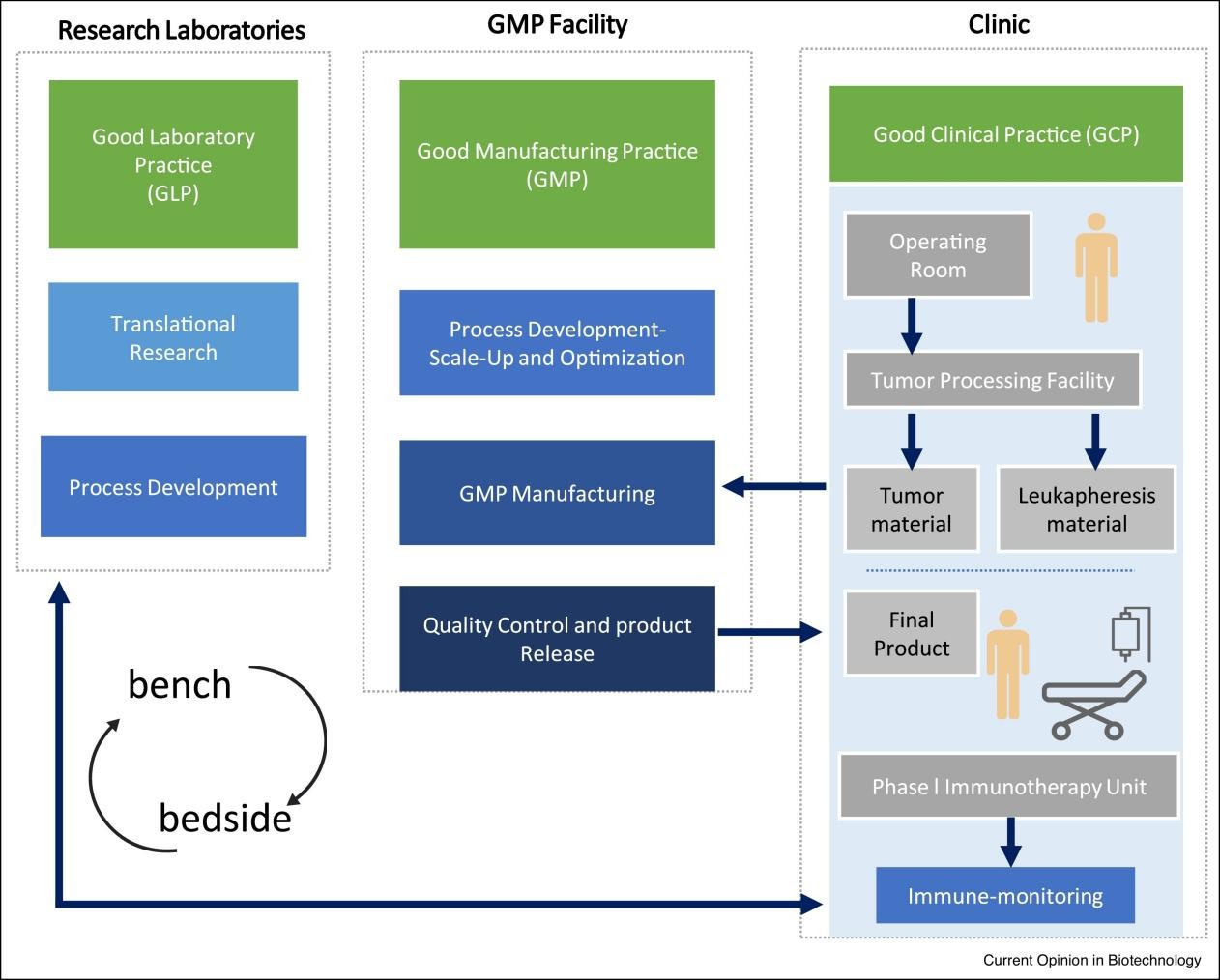 Fig.3 Bench to bedside process flow6.
Fig.3 Bench to bedside process flow6.
As the demand for advanced therapies grows, the future of cell therapy lies in making it scalable, standardized, and globally accessible7. Today's manufacturing processes are often labor-intensive, customized, and costly—especially for autologous treatments. But that's changing. The field is steadily moving from manual workflows to automated systems, and from fully personalized therapies toward universal "off-the-shelf" cell products.
One major goal is the development of allogeneic cell therapies that can be manufactured in large batches, frozen, stored, and used for any compatible patient—much like traditional medicines. These therapies promise to significantly reduce costs, speed up delivery, and simplify logistics.
To support this transition, several cutting-edge technologies are emerging:
These innovations are not just technical upgrades—they represent a shift toward democratizing cell therapy, making it available to more patients, in more places, at a lower cost.
In the future, cell therapies may become as common and accessible as vaccines or antibiotics are today. By integrating automation, standardization, and digital tools into GMP manufacturing, we are paving the way for a new era in medicine—where living cells become affordable cures for millions around the world.
Cell therapy is often hailed as the future of medicine—but its success depends on more than just scientific breakthroughs7. The real power of these living therapies lies in the precision and reliability of their production. While discovery happens in the lab, true impact happens only when those discoveries are transformed into safe, consistent, and scalable treatments that reach real patients.
That's where GMP plays a defining role. GMP turns hope into healing by ensuring that every dose of cell therapy—no matter how complex—is made under the highest standards of quality and safety. It's what ensures that a batch of genetically modified immune cells in a cleanroom can become a life-saving infusion for a cancer patient. It's what allows personalized medicine to leave the lab and enter the clinic.
In the end, the promise of cell therapy isn't just about what cells can do—it's about how well we prepare them to do it. Quality, consistency, and trust are not just technical goals; they are the foundations of a new kind of cure. With GMP as its amedicine, one patient at a time.
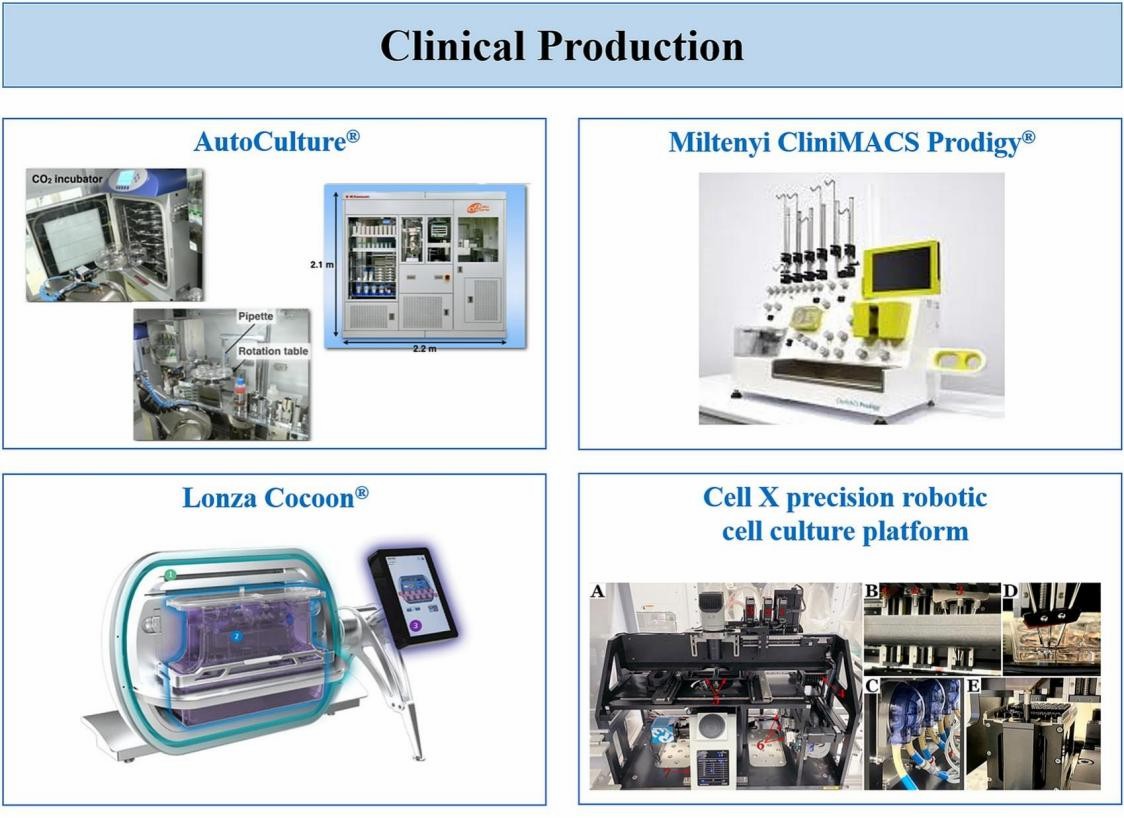 Fig.4 Images of systems automating multiple steps of the cell manufacturing proces7.
Fig.4 Images of systems automating multiple steps of the cell manufacturing proces7.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION