Inside CAR-T Cells: How Scientists Transform Immune Cells into Cancer-Fighting Heroes
Online Inquiry
What Are CAR-T Cells?
In the rapidly evolving landscape of cancer immunotherapy, CAR-T cells have emerged as a groundbreaking therapeutic approach that harnesses the power of a patient's own immune system to fight cancer. At their core, these cells represent a sophisticated fusion of cellular and genetic engineering, where T lymphocytes are modified to express a chimeric antigen receptor (CAR) - a synthetic receptor that combines the specificity of an antibody with the cytotoxic capabilities of T cells.
The journey of CAR-T cell therapy development began in the late 1980s at the Weizmann Institute of Science, but it wasn't until the past decade that we witnessed its true clinical potential. The fundamental principle behind this therapy lies in its ability to overcome one of cancer's most effective defense mechanisms: the evasion of immune recognition. By engineering T cells to express CARs, we create immune cells that can recognize specific tumor antigens independent of Major Histocompatibility Complex (MHC) presentation - a limitation that often hampers natural T cell responses.
The structure of a CAR is elegantly complex, typically comprising four distinct domains:
-
An extracellular antigen-binding domain, usually derived from an antibody's single-chain variable fragment (scFv)
-
A hinge region that provides flexibility and optimal spatial orientation
-
A transmembrane domain anchoring the receptor to the cell membrane
-
Intracellular signaling domains that activate T cells upon antigen recognition
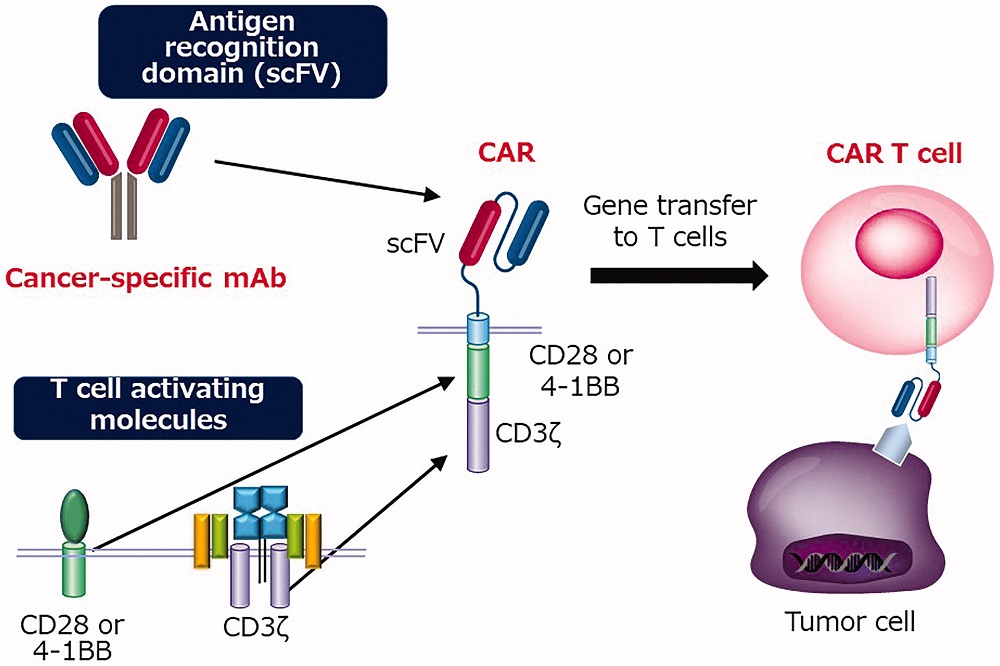 Fig.1 Illustration of the structure of CAR and CAR-T cell1,5.
Fig.1 Illustration of the structure of CAR and CAR-T cell1,5.
The evolution of CAR design has progressed through multiple generations, each addressing specific limitations of its predecessors. First-generation CARs contained only the CD3ζ signaling domain, while second-generation constructs incorporated co-stimulatory domains like CD28 or 4-1BB. Third-generation CARs feature multiple co-stimulatory domains, and fourth-generation variants, also known as TRUCKs (T cells Redirected for Universal Cytokine Killing), can express additional molecules like cytokines or suicide genes for enhanced functionality and safety.
How Are CAR-T Cells Made?
The CAR-T cell production process represents a complex interplay of cellular biology, genetic engineering, and quality control measures. The CAR-T therapy manufacturing process typically spans 2-3 weeks and involves several critical steps that must be executed with precision to ensure product safety and efficacy.
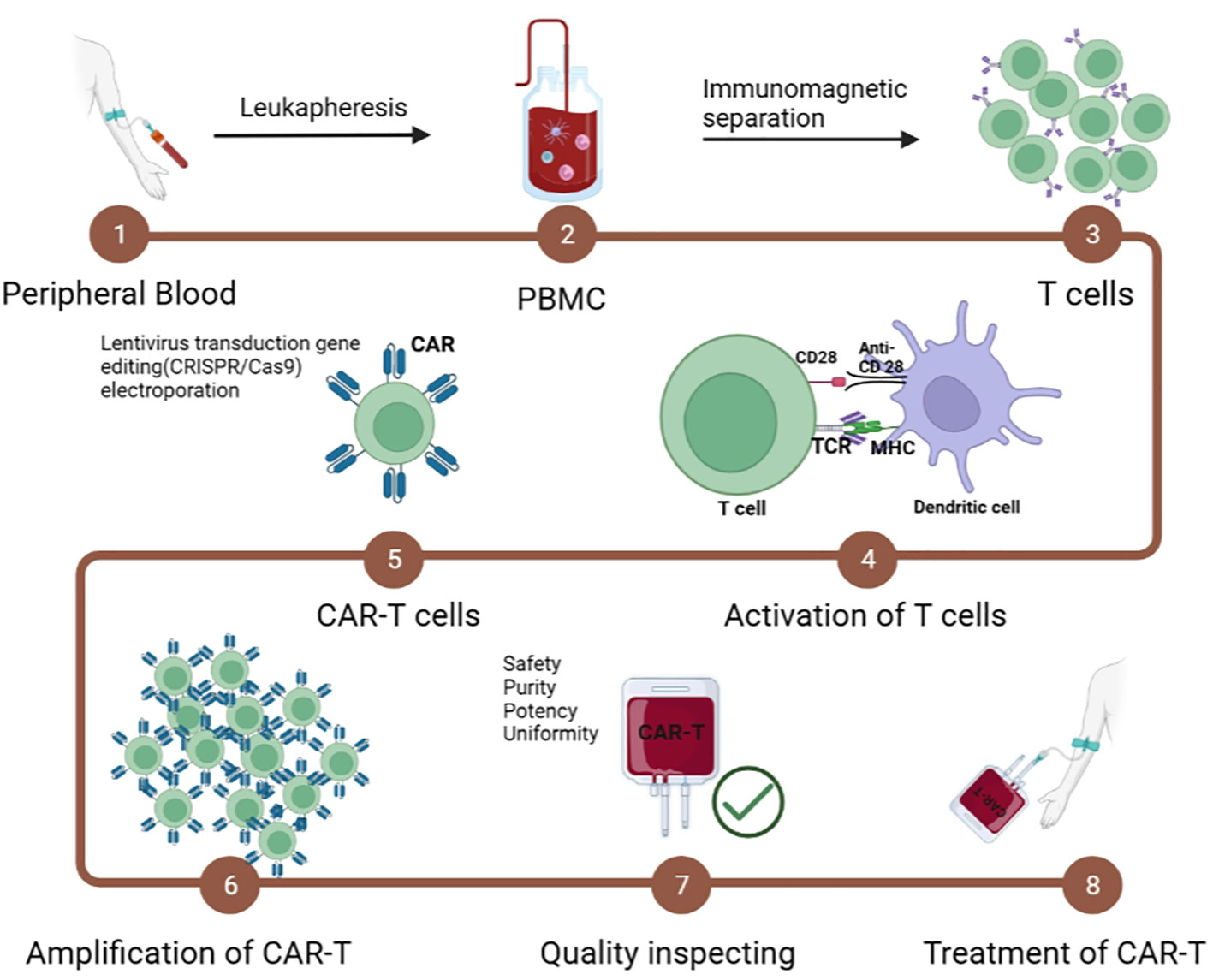 Fig.2 Manufacturing steps of CAR-T cells2,5.
Fig.2 Manufacturing steps of CAR-T cells2,5.
Patient Material Collection and T Cell Isolation
The manufacturing journey begins with leukapheresis, where a patient's white blood cells are collected while returning other blood components to the circulation. This process typically takes 3-4 hours and requires careful timing to ensure optimal T cell collection. The collected product undergoes density gradient centrifugation to isolate peripheral blood mononuclear cells (PBMCs), followed by specific T cell enrichment using magnetic bead-based selection or other separation techniques.
T Cell Activation and Expansion
A crucial step in the CAR-T cell production protocol involves T cell activation, traditionally accomplished using anti-CD3/CD28 antibodies conjugated to magnetic beads or artificial antigen-presenting cells. This activation creates an optimal environment for subsequent genetic modification and expansion. The choice of activation method significantly impacts the final product's phenotype and functionality.
Modern expansion protocols utilize specialized bioreactors that maintain precise control over:
-
Temperature (37°C ± 0.5°C): Precise temperature control is crucial for optimal T cell proliferation and metabolism while preventing heat stress responses
-
CO2 concentration (5% ± 0.2%): Maintains proper pH buffering and cellular respiration essential for T cell function
-
pH (7.2-7.4): Critical for enzyme activity and cellular signaling pathways that regulate T cell activation and expansion
-
Oxygen tension (40-60%): Balances metabolic demands while avoiding oxidative stress that could compromise cell viability
-
Nutrient availability: Ensures sufficient glucose, amino acids, and growth factors for sustained cell growth and optimal CAR expression
Genetic Engineering Strategies
The introduction of the CAR construct into T cells represents one of the most critical steps in the manufacturing process. Two primary approaches dominate the field:
1. Viral Vectors:
-
Lentiviral vectors: Offer stable integration and can transduce both dividing and non-dividing cells, with proven clinical efficacy in multiple approved CAR-T products
-
Retroviral vectors: Require cell division for integration but have a long safety track record and established regulatory pathway
-
Adenoviral vectors: Provide high transduction efficiency and large payload capacity, though typically resulting in transient expression due to non-integration, making them suitable for specific therapeutic strategies requiring temporary CAR expression
2. Non-viral Methods:
-
Transposon systems (e.g., Sleeping Beauty, PiggyBac): Offer cost-effective gene transfer with large payload capacity and stable integration, particularly beneficial for multiplexed CAR designs and complex engineering strategies
-
CRISPR-Cas9 mediated insertion: Enables precise genomic integration at predetermined loci, reducing the risk of insertional mutagenesis and allowing for targeted gene knockout/knock-in strategies
-
DNA electroporation: Provides a straightforward and rapid approach for transient CAR expression, using electrical pulses to temporarily permeabilize cell membranes for DNA delivery
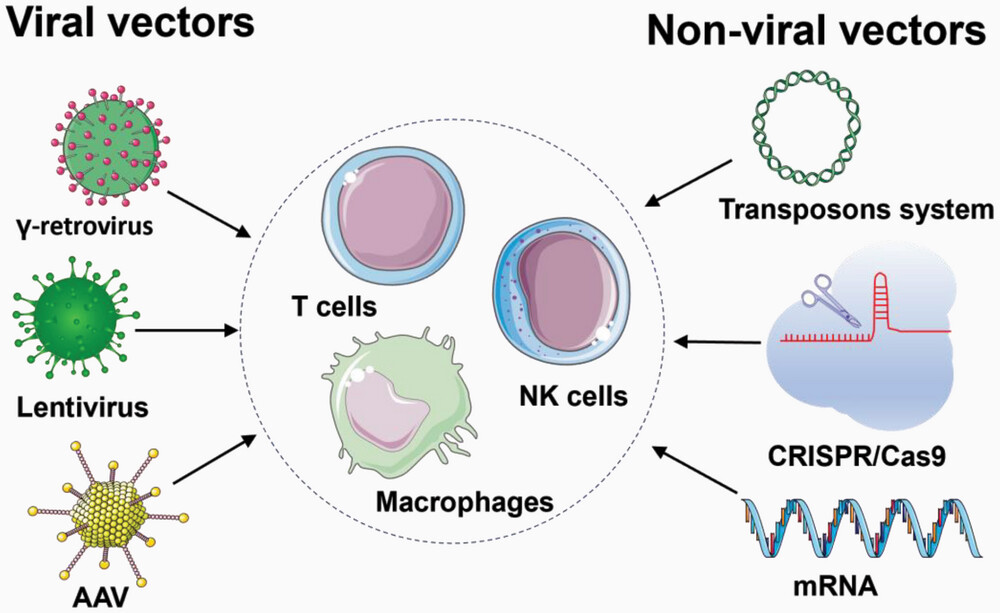 Fig.3 Manufacturing steps of CAR-T cells3,5.
Fig.3 Manufacturing steps of CAR-T cells3,5.
Quality Control and Release Testing
Before release, CAR-T cell products undergo rigorous testing to ensure safety and potency:
1. Identity Testing:
-
Flow cytometry analysis of CAR expression: Quantifies the percentage of CAR-positive cells and expression levels per cell
-
T cell subset composition: Determines the ratio of CD4+ to CD8+ T cells and ensures balanced population distribution
-
Memory/effector phenotype distribution: Assesses the presence of different T cell subsets crucial for both immediate and long-term anti-tumor responses
2. Purity Assessment:
-
Residual activation beads: Ensures complete removal of stimulation beads that could cause adverse effects in patients
-
Host cell proteins: Quantifies remaining non-T cell proteins that might trigger immunogenic responses
-
Process-related impurities: Measures levels of reagents used during manufacturing that must be below safety thresholds
3. Potency Evaluation:
-
Cytokine production profile: Measures key cytokines like IFN-γ and IL-2 to confirm functional activation capability
-
Specific target cell killing capacity: Determines the efficiency and speed of tumor cell elimination in controlled assays
-
CAR-mediated signaling functionality: Verifies proper signal transduction upon antigen recognition
4. Safety Testing:
-
Sterility: Confirms absence of bacterial and fungal contamination through multiple culture methods
-
Mycoplasma: Screens for these difficult-to-detect contaminants that can affect cell function
-
Endotoxin levels: Ensures bacterial endotoxin concentrations are below pyrogenic thresholds
-
Replication-competent viral testing (for viral vector-modified products): Verifies absence of infectious viral particles
The manufacturing process must be carefully documented and validated according to Good Manufacturing Practice (GMP) guidelines. This includes:
-
Environmental monitoring
-
Raw material testing and release
-
In-process controls
-
Final product specifications
-
Stability studies
-
Chain of custody documentation
Recent Innovations in Manufacturing
The field of CAR-T cell production continues to evolve rapidly. Several innovative approaches are being developed to enhance manufacturing efficiency and product quality:
1. Automated Manufacturing Platforms:
-
Closed system operations: Maintains sterility throughout the entire manufacturing process while reducing clean room requirements
-
Reduced operator intervention: Minimizes human error and variability while increasing process consistency
-
Improved reproducibility: Ensures batch-to-batch consistency through standardized automated protocols
-
Decreased contamination risk: Eliminates exposure to environmental contaminants through sealed processing units
2. Novel Selection Methods:
-
Microfluidic-based cell sorting: Enables gentle and precise separation of specific T cell subsets based on multiple parameters
-
Antibody-free separation techniques: Reduces costs and potential immunogenicity while maintaining high purification efficiency
-
Real-time monitoring systems: Provides continuous assessment of cell quality and process parameters for immediate optimization
3. Advanced Culture Systems:
-
Perfusion bioreactors: Maintains optimal nutrient levels while removing waste products for enhanced cell growth
-
Gas-permeable culture vessels: Improves oxygen delivery and reduces cell stress during expansion
-
3D culture platforms: Better mimics physiological conditions and enhances cell-cell interactions
4. Alternative Genetic Modification Strategies:
-
Site-specific integration systems: Ensures precise CAR gene insertion while minimizing off-target effects
-
Novel non-viral delivery methods: Reduces manufacturing costs and simplifies regulatory compliance
-
Improved vector designs: Enhances transduction efficiency and CAR expression stability
The successful implementation of these manufacturing processes requires a deep understanding of cellular biology, process engineering, and regulatory requirements. Continuous process improvement and optimization are essential for advancing the field and making CAR-T cell therapy more accessible to patients.
Critical considerations for process optimization include:
-
Minimizing manufacturing time
-
Reducing costs
-
Improving product consistency
-
Enhancing scalability
-
Maintaining product quality
-
Meeting regulatory requirements
As the field continues to mature, we can expect further refinements in manufacturing processes, leading to more efficient and cost-effective production methods while maintaining the highest standards of product quality and safety.
How Do CAR-T Cells Work?
The mechanism of action of CAR-T cells represents a sophisticated integration of synthetic biology and natural immune responses. Unlike conventional T cells that require antigen presentation via the MHC, CAR-T cells can directly recognize surface antigens through their engineered receptors, initiating a cascade of events that ultimately leads to target cell elimination.
Recognition and Activation Phase
When a chimeric antigen receptor encounters its specific target antigen on a cancer cell, several critical events occur in rapid succession:
1. Initial Binding:
-
The scFv domain of the CAR engages with the target antigen
-
Conformational changes in the receptor structure trigger initial signaling events
-
Multiple receptor-antigen interactions strengthen the immunological synapse
2. Signal Transduction:
-
The CD3ζ domain initiates primary activation signaling
-
Co-stimulatory domains (CD28/4-1BB) provide essential secondary signals
-
Downstream signaling cascades activate multiple pathways including: PI3K/AKT pathway, MAP kinase pathway, NFκB signaling, calcium-dependent pathways
Effector Functions
Activated CAR-T cells employ multiple mechanisms to eliminate target cells:
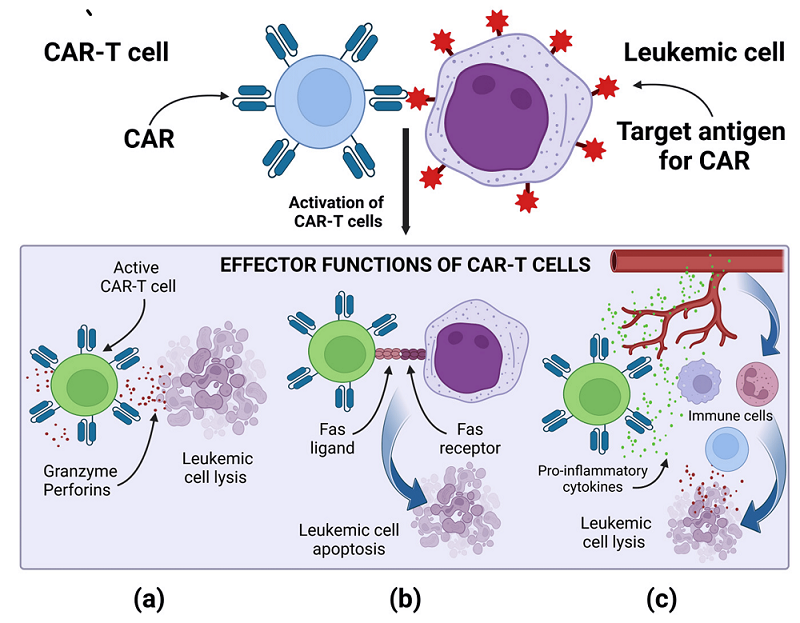 Fig.4 Effector mechanism of CAR-T cell4,5.
Fig.4 Effector mechanism of CAR-T cell4,5.
1. Direct Cytotoxicity:
-
Release of perforin to form membrane pores: Creates transmembrane channels that disrupt tumor cell membrane integrity
-
Granzyme secretion leading to target cell apoptosis: Activates caspase-dependent cell death pathways once inside target cells
-
FAS-FASL interaction triggering death receptor pathways: Initiates extrinsic apoptotic cascades through death receptor signaling
2. Cytokine Production:
-
IL-2 secretion for T cell proliferation and survival: Supports autonomous growth and sustained activation of CAR-T cells
-
IFN-γ production enhancing anti-tumor immune responses: Activates macrophages and increases MHC expression on tumor cells
-
TNF-α release contributing to tumor cell death: Triggers inflammatory responses and direct tumor cell apoptosis
-
GM-CSF secretion recruiting additional immune cells: Attracts and activates dendritic cells and neutrophils to the tumor site
3. Proliferation and Persistence:
-
Rapid clonal expansion at tumor sites: Generates large numbers of tumor-specific effector cells within days
-
Formation of memory CAR-T cells: Establishes long-lived memory populations for sustained anti-tumor immunity
-
Long-term surveillance against tumor recurrence: Maintains vigilant monitoring for potential cancer cell reemergence
Regulatory Mechanisms
CAR-T cell activity is regulated through various intrinsic and extrinsic mechanisms:
1. Inhibitory Receptors:
-
PD-1 expression modulating excessive activation
-
LAG-3 and TIM-3 providing additional regulatory checkpoints
-
CTLA-4 controlling proliferation rates
2. Metabolic Regulation:
-
Shift to glycolytic metabolism during activation
-
Oxidative phosphorylation in memory cell formation
-
Nutrient competition with tumor cells
3. Spatial Organization:
-
Migration to tumor sites guided by chemokine gradients
-
Formation of structured immune synapses
-
Tissue-specific homing patterns
Challenges and Adaptations
CAR-T cell activity is regulated through various intrinsic and extrinsic mechanisms:
The functionality of CAR-T cells faces several challenges in the tumor microenvironment:
1. Immunosuppressive Factors:
-
TGF-β exposure: Suppresses T cell proliferation and effector functions while promoting regulatory T cell development
-
Adenosine accumulation: Inhibits T cell activation and promotes anergy through A2A receptor signaling
-
IDO expression by tumor cells: Depletes tryptophan and generates immunosuppressive metabolites
-
Regulatory T cell presence: Actively suppresses CAR-T cell function through multiple inhibitory mechanisms
2. Physical Barriers:
-
Dense stromal architecture: Impedes CAR-T cell infiltration and creates immunosuppressive niches
-
Hypoxic conditions: Alters T cell metabolism and reduces effector functions
-
Altered pH environment: Disrupts cellular signaling and impairs cytokine production
-
Limited nutrient availability: Compromises T cell function through metabolic restrictions
To address these challenges, newer generations of CAR-T cells incorporate additional features:
1. Enhanced Trafficking:
-
Expression of chemokine receptors
-
Modified adhesion molecules
-
Improved tissue penetration capabilities
2. Resistance to Inhibition:
-
Dominant-negative receptors
-
Checkpoint resistance modifications
-
Enhanced metabolic fitness
3. Improved Safety:
-
Inducible suicide genes
-
Logic-gated activation
-
Titratable expression systems
The complex interplay between CAR-T cells and their targets continues to be an area of active research, with new insights regularly emerging about optimization strategies and potential combination approaches with other therapeutic modalities.
References
-
Hosen, Naoki. "CAR T cell therapy." Immunological Medicine 44.2 (2021): 69-73.
-
Chen, Ran, et al. "CAR-T treatment for cancer: prospects and challenges." Frontiers in Oncology 13 (2023): 1288383.
-
Chen, Zhaozhao, Yu Hu, and Heng Mei. "Advances in CAR‐Engineered Immune Cell Generation: Engineering Approaches and Sourcing Strategies." Advanced Science 10.35 (2023): 2303215.
-
Zarychta, Julia, et al. "CAR-T cells immunotherapies for the treatment of acute myeloid leukemia—recent advances." Cancers 15.11 (2023): 2944.
-
Distributed under Open Access license CC BY 4.0, without modification.
 Fig.1 Illustration of the structure of CAR and CAR-T cell1,5.
Fig.1 Illustration of the structure of CAR and CAR-T cell1,5.
 Fig.2 Manufacturing steps of CAR-T cells2,5.
Fig.2 Manufacturing steps of CAR-T cells2,5.
 Fig.3 Manufacturing steps of CAR-T cells3,5.
Fig.3 Manufacturing steps of CAR-T cells3,5.
 Fig.4 Effector mechanism of CAR-T cell4,5.
Fig.4 Effector mechanism of CAR-T cell4,5.
 NEWSLETTER
NEWSLETTER
 NEW SOLUTION
NEW SOLUTION
 NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
 NEW SOLUTION
NEW SOLUTION