Diabetes, a chronic disease once considered to require lifelong management, is at a major turning point in the treatment landscape. More than 537 million people worldwide (International Diabetes Federation 2024 data) are chronically dependent on insulin injections and medications to control their blood sugar, and are under constant threat of complications such as hypoglycemic coma, kidney failure, and blindness. Conventional treatments treat the symptoms but not the root cause, and the emergence of cellular therapies has brought unprecedented hope for revolutionizing the diabetes treatment paradigm from the source.
The core cause of type 1 diabetes lies in an erroneous attack by the body's immune system. The patient's immune system recognizes the beta cells responsible for insulin production in their own pancreas as a foreign threat and destroys them, resulting in an absolute lack of insulin production. This autoimmune response is usually triggered by a combination of genetic predisposition and certain environmental triggers, such as specific viral infections, and often develops in childhood or adolescence. Due to the absolute insulin deficiency that results from its etiology, patients are at risk for serious acute complications, particularly diabetic ketoacidosis (DKA). When the body is unable to utilize blood sugar for energy due to a lack of insulin, it turns to breaking down fats in large quantities, producing a toxic accumulation of ketone bodies that can lead to acidosis, dehydration, coma, and even death, which is a life-threatening emergency. In addition, insulin therapy itself can trigger severe hypoglycemia. Over the long term, persistent hyperglycemia damages tiny blood vessels and nerves throughout the body, leading to a series of chronic complications: diabetic retinopathy is one of the leading causes of blindness in adults; diabetic nephropathy can progress to kidney failure requiring dialysis or transplantation; and there is a significantly increased risk of heart disease, stroke, and peripheral artery disease, which can have a serious impact on quality of life and life expectancy.
Type 2 diabetes, on the other hand, stems primarily from insulin resistance accompanied by a progressive relative deficiency in insulin secretion. Initially, the body's cells are resistant to insulin, making it ineffective in transporting blood glucose into the cells for utilization. As a substitute, pancreatic beta cells overload to secrete more insulin. However, over time, under the stress of high demand and genetic factors, the beta cells fail and are unable to secrete enough insulin to overcome the resistance, resulting in elevated blood glucose. Genetic background is fundamental, but environmental and lifestyle factors such as obesity, physical inactivity, and unhealthy diet are key triggering and driving forces. Acute complications include hyperglycemic hyperosmolar state. Chronic complications are similar to those of type 1 and include retinopathy, which causes blindness; nephropathy, which causes kidney failure; and neuropathy, which causes pain, sensory abnormalities, and dysfunction.
Although traditional therapies can temporarily control blood glucose or enhance the response of some tissues to insulin, they cannot reach the core of the disease. They can neither truly reverse the key problem that leads to uncontrolled blood glucose, nor can they prevent or repair the inevitable failure of pancreatic β-cells, which is essentially "treating the symptoms but not the root cause".
Long-term reliance on traditional treatments puts patients at serious risk of multi-organ complications. Continued damage to blood vessels and nerves from hyperglycemia can lead to retinopathy (blindness), kidney failure (requiring dialysis or kidney transplantation), diabetic foot (which can lead to amputation), and cardiovascular disease (e.g., heart failure, myocardial infarction), which can seriously reduce quality of life, and can lead to disability or even death.
Frequent insulin injections or medications place a significant physical and mental burden on patients. This day-to-day treatment pattern easily triggers burnout and resistance, and as the duration of the disease increases, the patient's patience gradually depletes, resulting in a significant decrease in treatment cooperation and long-term compliance, which affects the effectiveness of treatment1.
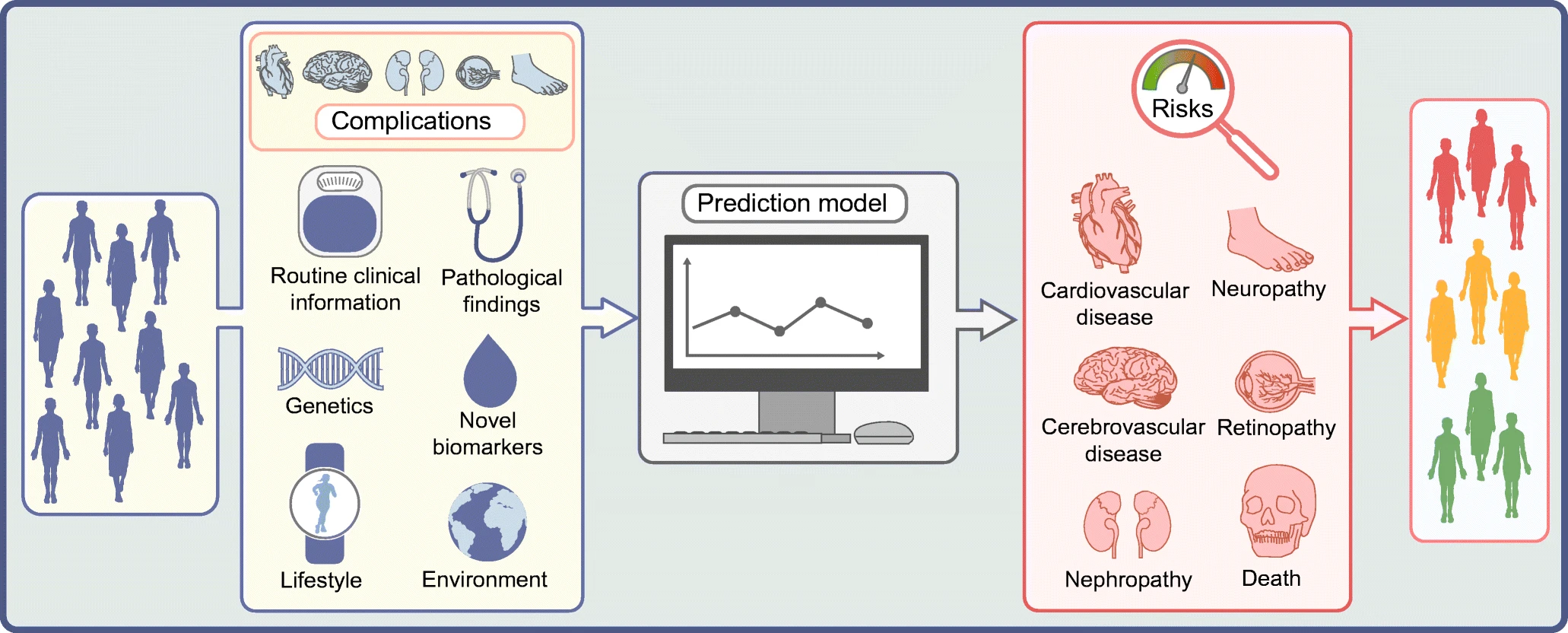 Fig.1 Causes and complications of diabetes1
Fig.1 Causes and complications of diabetes1
The core idea of cellular therapy goes straight to the root of diabetes: by replenishing or repairing damaged pancreatic beta cells in the body, it rebuilds the body's natural ability to regulate blood sugar on its own.
In a healthy pancreas, pancreatic beta cells act as smart factories, precisely sensing changes in blood glucose and secreting the right amount of insulin, directing the cells to take up glucose. in type 1 diabetes, the immune system mistakenly attacks and destroys these factories; and in the later stages of type 2 diabetes, these same factories fail from chronic overload.
Stem cell therapy: Stem cell therapy is dedicated to harnessing the powerful differentiation potential of pluripotent stem cells (including embryonic stem cells or induced pluripotent stem cells obtained by reprogramming the patient's own cells) in vitro, and inducing them to differentiate into functionally mature pancreatic β-cells or islet-like structures through precise culture conditions. The goal of these new cells, which are "custom-produced" in the laboratory, is that when transplanted back into the patient, they will be able to sense changes in blood glucose and precisely secrete insulin, thus re-establishing the body's natural glucose regulation system. The central attraction of this pathway is that it provides a theoretically "limitless" source of cells and is expected to solve the problem of immune rejection through the use of the patient's own cells, representing one of the most promising directions for achieving a functional cure for diabetes in the future.
Islet cell transplantation: Islet cell transplantation was one of the earliest practices of the "cell replacement" strategy, in which healthy islet cells isolated and purified from the pancreas of a deceased donor were transplanted into patients, usually with type 1 diabetes, as a direct replacement for their destroyed islet β-cells. The historic contribution of this approach is the conclusive demonstration that functional β-cell transplantation is effective in restoring endogenous insulin secretion, significantly improving glycemic control and reducing the risk of severe hypoglycemia, and even weaning some patients off exogenous insulin. However, its widespread use is limited by two fundamental constraints: the scarcity of donor organs to meet the huge demand, and the need for lifelong use of potent immunosuppressive drugs to prevent rejection (and in the case of type 1 diabetes to suppress autoimmune relapses), which come with a significant risk of side effects, and are currently reserved for a select group of high-risk patients2.
Immune cell modulation therapy: Immune cell modulation therapy, on the other hand, focuses on autoimmune attack, the core cause of type 1 diabetes. The goal is not to replace cells, but to correct or inhibit the body's faulty immune response in order to preserve the patient's residual beta-cell function or to create a safe in vivo environment for other cell transplants (e.g., stem cell or islet transplants). Strategies include the in vitro expansion and reinfusion of the patient's own regulatory T cells to enhance immune tolerance, the use of engineered T cells or antibodies to specifically remove disease-causing immune cells attacking the islets, and the use of modified antigen-presenting cells to induce immune tolerance. These therapies are aimed at "treating the disease at its source" by restoring immune tolerance to islet β-cells, and have the advantage of being highly targeted, either alone to slow disease progression or protect residual function, or in combination with other cellular therapies to protect the transplanted cells from attack.
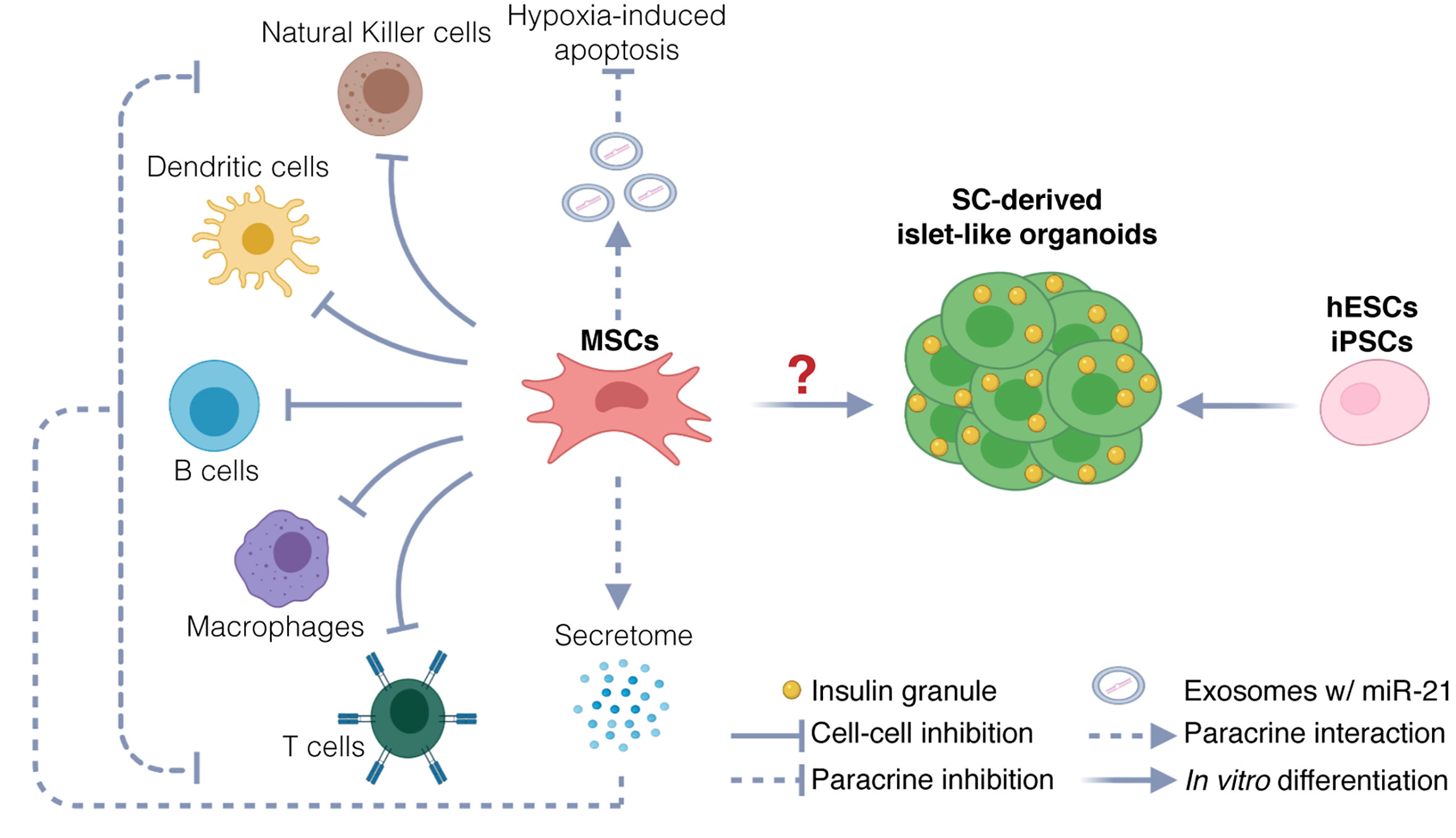 Fig.2 Possible Mechanisms of Mesenchymal Stem Cell (MSC) Therapy for Type 1 Diabetes Mellitus3
Fig.2 Possible Mechanisms of Mesenchymal Stem Cell (MSC) Therapy for Type 1 Diabetes Mellitus3
The core mechanism of stem cell therapy for diabetic foot is to repair damaged tissues through multi-target synergy. First, stem cells directly secrete key factors such as vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF), activate signaling pathways such as PI3K/Akt, and significantly promote vascular neogenesis to improve local ischemia; at the same time, by regulating the polarization of macrophages to the anti-inflammatory M2 type, increasing the proportion of regulatory T cells (Tregs), and inhibiting pro-inflammatory factors (e.g., TNF-α, IL-6), the immune microenvironment is remodeled to reduce chronic inflammation. ), remodeling the immune microenvironment and reducing chronic inflammation.
Second, stem cells repair nerve damage and improve nerve blood supply through nerve growth factor (NGF) and neurotrophic factor (NT-3); and promote collagen deposition and tissue remodeling by activating fibroblasts and regulating matrix metalloproteinases. In addition, its paracrine exosomes carry miRNAs (e.g. miR-21-5p) to further enhance angiogenesis and anti-inflammatory effects. These mechanisms synergistically overcome the repair obstacles of the diabetic hyperglycemic microenvironment and accelerate ulcer healing4.
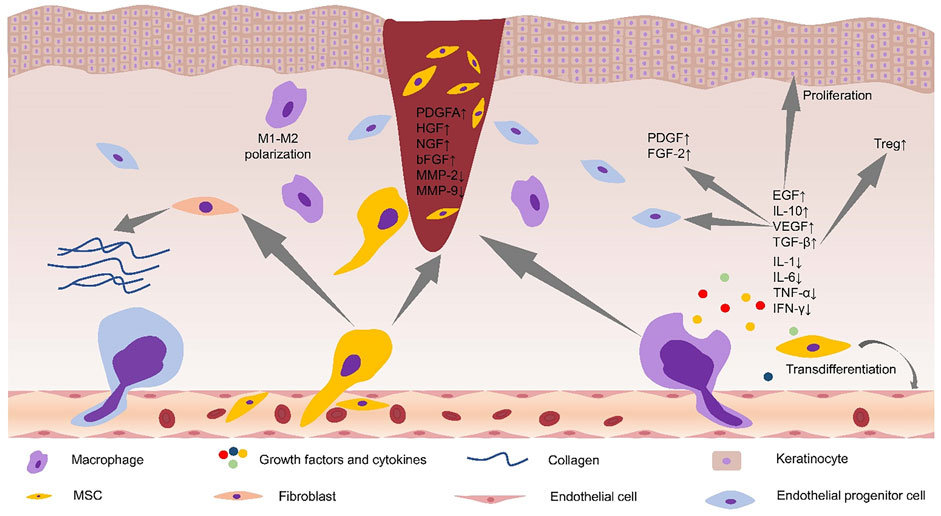 Fig.3 Alterations in the microenvironment of diabetic foot ulcers after stem cell application4.
Fig.3 Alterations in the microenvironment of diabetic foot ulcers after stem cell application4.
The core mechanism of MSCs in the treatment of diabetic cardiomyopathy (DCM) lies in their powerful paracrine effects. The bioactive substances secreted by MSCs can intervene in the pathological process of DCM at multiple targets: significantly inhibiting myocardial inflammatory responses (e.g., lowering TNF-α, IL-6), attenuating oxidative stress, inhibiting myocardial fibrosis (e.g., down-regulation of TGF-β/Smad signaling, (e.g., downregulation of TGF-β/Smad signaling, reduction of collagen deposition), counteracting cardiomyocyte apoptosis, and promoting neovascularization (e.g., increase of VEGF, improvement of perfusion), leading to a comprehensive improvement of cardiac function. In preclinical animal models, MSCs of either bone marrow, adipose or placental origin can effectively reduce myocardial hypertrophy and fibrosis, and improve cardiac function indexes.
In terms of clinical application, preliminary studies have shown that MSCs transplantation is safe and feasible in patients with various cardiomyopathies combined with diabetes mellitus, and can improve cardiac function and quality of life. However, the difficulty of early diagnosis of DCM, the diabetic environment that impairs the function of autologous MSCs, and the lack of large-scale clinical trials specifically targeting DCM are the main challenges. Optimization of cell sources, delivery strategies, and standardized preparation processes are needed for the future5.
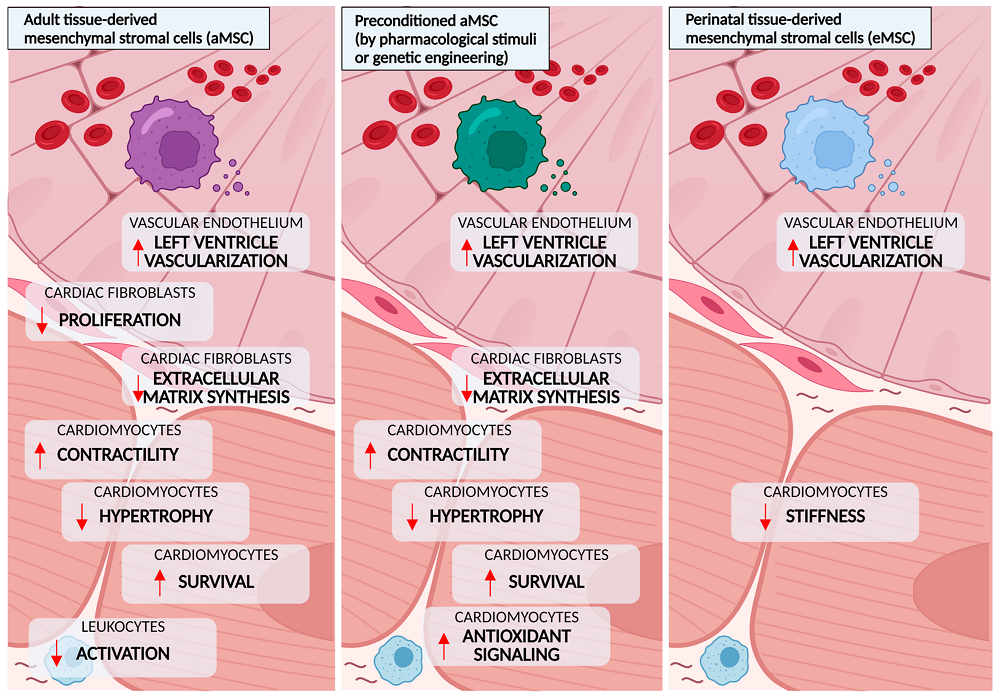 Fig.4 Beneficial effects of mesenchymal stromal cells (MSCs) observed in preclinical models of diabetes5.
Fig.4 Beneficial effects of mesenchymal stromal cells (MSCs) observed in preclinical models of diabetes5.
Despite the bright future, key challenges still need to be overcome for cell therapy to truly benefit the majority of diabetic patients.
Both stem cells and donor-derived islet cells are recognized by the patient's immune system as "outsiders" and attacked. Traditional anti-rejection drugs have significant side effects. Scientists are using gene editing to remove the "identity tags" on the surface of the cells to make them "invisible", or they are using special biomaterials to wrap the cells to form a physical "barrier" that allows the entry and exit of nutrients but prevents the immune system from attacking them. nutrients in and out but blocking immune attacks, while working to develop "universal" stem cell banks that do not require strict mating.
The second core obstacle is ensuring the survival and function of transplanted cells. Transplanted cells need to "settle" successfully and work efficiently in their new environment. Researchers are working to optimize the microenvironment at the transplant site, for example, by using scaffolds that mimic the structure of the natural pancreas to support the cells, and by focusing on angiogenesis, which promotes the rapid establishment of a rich network of blood vessels at the transplant site to ensure that the cells receive sufficient oxygen and nutrients, and that insulin secreted by the cells enters the bloodstream in a timely manner to do its job.
High cost and accessibility are important divides that prevent widespread adoption. Current cell therapy development and production processes are extremely complex, making single treatments extremely expensive. The key to lowering costs is to scale up and automate the production of stem cell culture and differentiation to increase efficiency, and to continue to innovate and optimize the process to improve success rates.
In the next 3-5 years, the focus of diabetes cell therapy will be on breaking through bottlenecks and improving accessibility. In the next 3-5 years, the focus of diabetes cell therapy will be on breaking through bottlenecks and improving accessibility. The primary goal is to promote the release of "universal" cell products that do not require strict matching and can solve the problem of immune rejection. At the same time, we will continue to optimize the efficacy, durability and stability of our therapies and explore the application of our therapies to a wider range of diabetes patient types, such as complex and difficult-to-manage cases.
In the longer term, cell therapy will be deeply integrated with cutting-edge biotechnology, such as gene editing and tissue engineering, in order to develop a "bio-artificial pancreas" with stronger functions and longer lifespan. Artificial intelligence will also be deeply empowered to analyze individual genetic, immune and metabolic characteristics to customize the most accurate and lowest-risk cell therapy program for each patient.
From a reliance on "lifelong management" to a potential "one-time cure," cell therapy is reshaping the landscape of diabetes care at an unprecedented rate. It represents not only the rise of a revolutionary medical technology, but also the light of hope for millions of patients around the world to break free from the disease and regain their health.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION