Macrophages are phagocytes widely distributed in various tissues, they can recognize, engulf and degrade pathogens and cellular debris, and participate in innate and adaptive immune responses. There are two developmental sources of macrophages: one is developed from hematopoietic stem cells in the bone marrow to monocytes, migrates to tissues and then differentiates into tissue-specific macrophages; the other is in the yolk sac, fetal Embryonic regions near the liver or dorsal aorta develop as resident macrophages. The differentiation and function of macrophages are influenced by a variety of factors, including signaling molecules, growth factors, transcription factors, epigenetic and post-transcriptional mechanisms and changes, and niche signaling. According to their function and activation status, macrophages can be divided into two subtypes: classically activated or M1-type macrophages and alternatively activated or M2-type macrophages. M1-type macrophages mainly have pro-inflammatory, anti-bacterial and anti-tumor effects, they can be polarized by lipopolysaccharide (LPS) alone or in combination with Th1 cytokines (such as IFN-γ, GM-CSF), and produce pro-inflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, IL-12, IL-23 and TNF-α; M2 type ,acrophages mainly have anti-inflammatory, immune regulation and tissue repair effects, and they can be stimulated by Th2 cytokines IL-4 and IL-13 polarize and produce anti-inflammatory cytokines such as IL-10 and TGF-β.
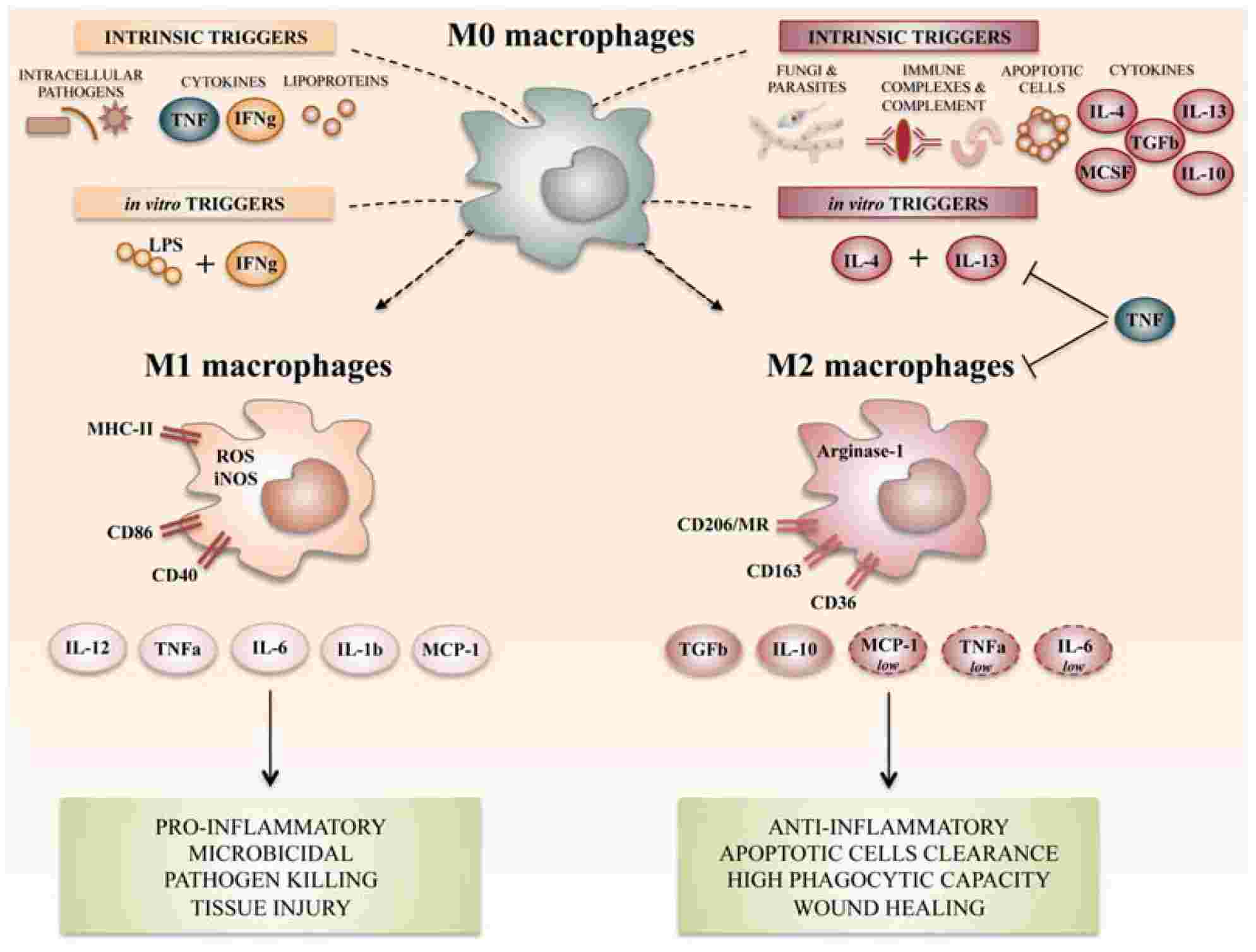 Fig 1 Summary of the main macrophage polarization states of activated macrophages. (Atri, 2018)
Fig 1 Summary of the main macrophage polarization states of activated macrophages. (Atri, 2018)
Adoptive transfer is an experimental method in which immune cells that have been manipulated or engineered outside the body are transplanted into another individual to observe their behavior and effects in vivo. Adoptive transfer can be used to study the development, differentiation, migration, function and interaction of immune cells. Adoptive transfer of macrophages refers to transplanting in vitro cultured or transgenic macrophages into another individual to explore their mechanism of action and therapeutic potential in different disease models. Adoptive transfer of macrophages is an important research direction, because it can reveal the key role of macrophages in infection, tumor, autoimmune diseases, etc., and provide a basis for the development of new immunotherapy strategies.
The principle of adoptive cell transfer therapy is to take the patient's own immune cells out of the body, undergo in vitro treatment or genetic modification, so that they have a stronger ability to recognize and kill tumor cells, and then infuse them back into the patient's body to exert anti-tumor effects. This approach can overcome the mechanism by which tumor cells escape the surveillance and clearance of the immune system, enhance the activity and persistence of immune cells, and improve the therapeutic effect.
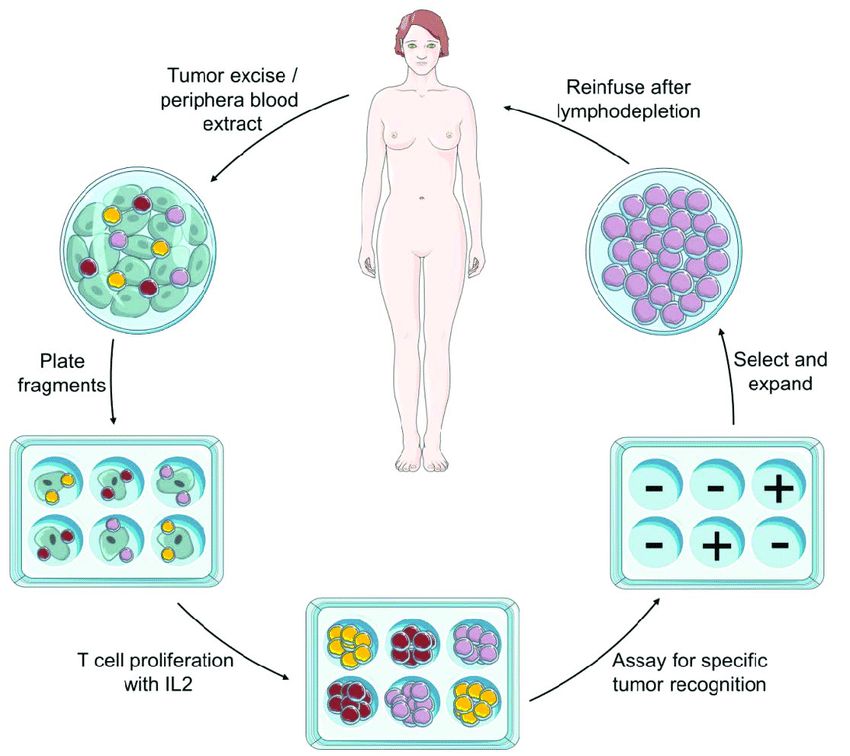 Fig.2 Schematic representation of adoptive cell transfer immunotherapy procedure. (Maria, 2015)
Fig.2 Schematic representation of adoptive cell transfer immunotherapy procedure. (Maria, 2015)
Currently, the following types of cells are used in Adoptive Cell Transfer Therapy:
Macrophage-based immunotherapeutic strategies have been developed, such as targeted clearance or reprogramming of TAMs, engineering modification, or adoptive transfer of macrophages. Macrophage adoptive transfer is a method of using cultured macrophages in vitro to treat cancer. Macrophages are immune cells that can phagocytize and kill tumor cells, but in the tumor microenvironment, they are often transformed by tumor factors into M2 macrophages that promote tumor growth and metastasis. Therefore, genetically engineering or otherwise modifying macrophages to have a persistent M1 phenotype and infusing them into patients is a potential tumor immunotherapy strategy.
The role of adoptive transfer of macrophages mainly includes the following aspects. Macrophages directly phagocytize and kill tumor cells and release cytotoxic factors such as NO and ROS. Macrophages, as antigen-presenting cells, activate and expand specific T cells to form an adaptive immune response. Macrophages secrete pro-inflammatory and anti-tumor cytokines, such as IL-12, TNF-α, etc., regulate the immune microenvironment, and recruit other immune effector cells, such as NK cells and DC cells. Macrophages remodel the phenotype and function of TAMs from M2 to M1.
The mechanism of adoptive transfer of macrophages mainly involves the following aspects. Source and differentiation of macrophages, such as bone marrow, peripheral blood, spleen, etc. Activation and polarization of macrophages, such as IFN-γ, LPS, IL-4, etc. Gene modification and expression of macrophages, such as CAR, TRAIL, CD40, etc. Interaction and signal transduction between macrophages and tumor cells or other immune cells, such as FcγR, TLR, CD47-SIRPα, etc.
The advantages of the adoptive transfer of macrophages are as follows:
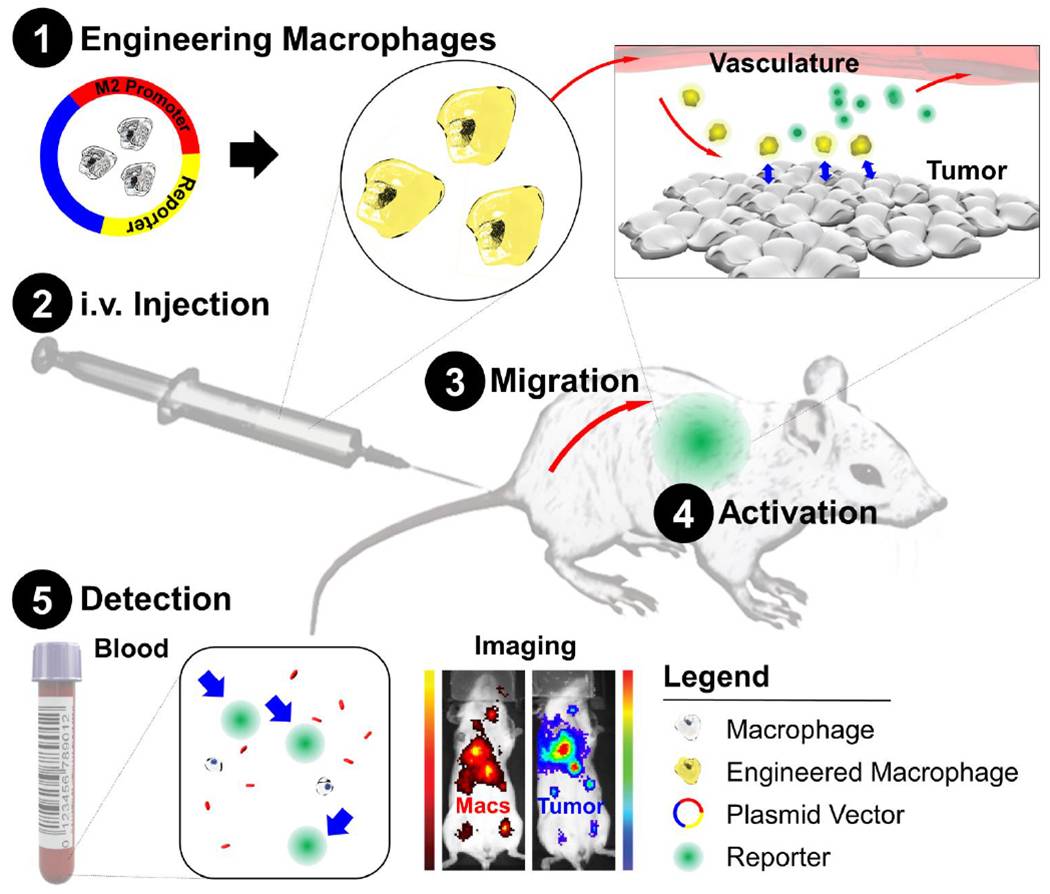 Fig.3 Schematic of diagnostic adoptive cell transfer. (Aalipour, 2019)
Fig.3 Schematic of diagnostic adoptive cell transfer. (Aalipour, 2019)
CAR-macrophage is a method of using genetic engineering technology to make macrophages express chimeric antigen receptors (CAR), which allows Macrophages to directly recognize and phagocytize tumor cells. The principle of CAR-macrophage is to remove the Macrophages of the patient or donor from the body, and through gene transfection, make it express a CAR molecule consisting of a single-chain antibody fragment that can recognize tumor-associated antigens and an Fc receptor that can transmit activation signals. These genetically modified Macrophages are then infused back into the patient, enabling them to directly recognize and phagocytose tumor cells.
Currently, the following types of CAR-macrophages are used for adoptive transfer of macrophages:
In addition to the above-mentioned types, there are other CAR-Macrophages targeting different tumor-associated antigens being developed and tested, such as CEA-CAR-macrophages, MUC1-CAR-macrophages, CD19-CAR-macrophages, etc. At present, CAR-macrophage, as a new type of immune cell therapy, has great potential and prospects, but it also faces some challenges and problems, such as the plasticity of macrophages, the inhibitory effect of tumor microenvironment, and the control of side effects. Therefore, further research and optimization are needed to improve the safety and efficacy of CAR-macrophage.
Empowered by our state-of-the-art CellRapeutics™ Chimeric Antigen Receptor (CAR) Technology, Creative Biolabs offers world-leading CAR-macrophage development services, aiming to improve the ability of CAR cells to attack solid tumors. Our one-stop services covering Target Identification & Selection, High-Affinity Antigen Binder Generation, CAR-MA Design & Construction, CAR-MA Preparation, Macrophages Activation and Expansion, In Vitro Assessments, Preclinical Tests and Clinical Trials. We also provide CAR-MA Vector Products, CAR-MA Cell Products, and a proprietary online system for customizing CAR and CAR cell products, which offers full options to meet all unique needs.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION