Macrophages are a type of mononuclear macrocytes widely distributed in various tissues, mainly derived from the bone marrow, and partly derived from the embryonic yolk sac or liver. Macrophages can undergo reversible phenotypic and functional changes depending on the surrounding microenvironmental factors, known as macrophage plasticity. Depending on the stimulus, macrophages can differentiate into two polarized phenotypes: M1 macrophages and M2 macrophages. M1 macrophages are activated by pro-inflammatory factors such as lipopolysaccharide (LPS) or Th1 cytokines (such as IFN-γ and GM-CSF), have strong antibacterial and anti-tumor capabilities, and can produce a large number of pro-inflammatory cytokines (such as IL -1β, IL-6, IL-12, IL-23, and TNF-α) and reactive oxygen species (ROS). M2 macrophages are activated by anti-inflammatory factors such as Th2 cytokines (such as IL-4 and IL-13), have the ability to promote tissue repair and angiogenesis, and can produce a large number of anti-inflammatory cytokines (such as IL-10, TGF- β) and mucopolysaccharides (GAGs). Under normal conditions, macrophages participate in physiological processes such as the body's immune defense, tissue homeostasis maintenance, and wound healing. In pathological situations, macrophages are also related to the development of many diseases, such as infection, autoimmune disease, obesity, atherosclerosis, neurodegenerative diseases, and tumor.
Since macrophages have important regulatory roles in the tumor microenvironment, monoclonal antibodies targeting macrophages are a promising strategy for tumor immunotherapy. Some macrophage surface molecules or secreted factors that can be used as targets have been discovered, such as CSF1/CSF1R, CD47/SIRPα, CD40, CCL2/CCR2, CXCR4, CD206, CD163, TGF-β, IL-12, etc. Monoclonal antibodies targeting these molecules can enhance tumor immune responses by clearing or recoding macrophages and have synergistic effects with other immunotherapeutic approaches (such as immune checkpoint inhibitors, adoptive cell transfer therapy, and tumor vaccines).
Table 1. Macrophage Surface Molecules or Secreted Factors as Targets
| Targets | Disease or condition | Monoclonal antibody |
|---|---|---|
| CSF1/CSF1R | Cancer | Emactuzumab, Cabiralizumab, Pexidartinib |
| CD47/SIRPα | Cancer | Hu5F9-G4, Magrolimab, CC-90002 |
| CD40 | Cancer | APX005M, Selicrelumab, Lucatumumab |
| CCL2/CCR2 | Cancer | PF-04136309, MLN1202, BMS-813160 |
| CXCR4 | Cancer | Ulocuplumab, Balixafortide, LY2510924 |
| CD206 | Cancer | RG7155, MOR107 |
| CD163 | Cancer | BI-204, BI-1206 |
| TGF-β | Cancer, inflammatory bowel disease | Fresolimumab, Galunisertib, LY2157299 |
| IL-12 | Cancer, atherosclerosis | ABT-874, Ustekinumab |
The colony-stimulating factor 1 (CSF1) or CSF1 receptor (CSF1/CSF1R) axis is a key signaling pathway regulating the survival, proliferation, differentiation, and function of macrophages. CSF1 is a cytokine mainly secreted by tumor cells and can bind to CSF1R on the surface of macrophages and activate downstream signaling pathways such as PI3K/AKT, MAPK/ERK and STAT3, thereby promoting the recruitment, survival and polarization of macrophages into M2 phenotype. The CSF1/CSF1R axis is highly expressed in a variety of solid tumors and is associated with tumor invasion, metastasis and poor prognosis. Monoclonal antibodies targeting the CSF1/CSF1R axis can inhibit tumor growth and spread by reducing the number and activity of tumor-associated macrophages (TAMs) by inhibiting the interaction between CSF1 and CSF1R.on the surface of macrophages and activate downstream signaling pathways such as PI3K/AKT, MAPK/ERK, and STAT3, thereby promoting the recruitment, survival, and polarization of macrophages into the M2 phenotype. The CSF1/CSF1R axis is highly expressed in a variety of solid tumors and is associated with tumor invasion, metastasis, and poor prognosis. Monoclonal antibodies targeting the CSF1/CSF1R axis can inhibit tumor growth and spread by reducing the number and activity of tumor-associated macrophages (TAMs) and inhibiting the interaction between CSF1 and CSF1R.
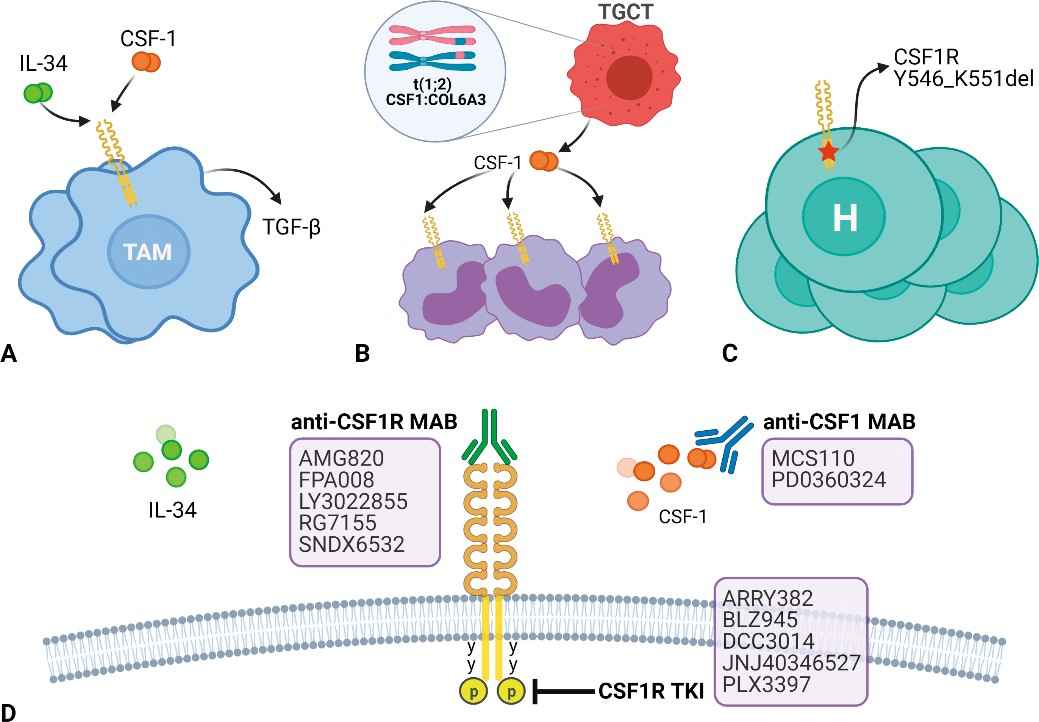 Fig.1 Pathogenic roles of CSF1/CSF1R
Fig.1 Pathogenic roles of CSF1/CSF1R
Several monoclonal antibodies that target the CSF1/CSF1R axis have advanced to clinical trials, such as RG7155 (Emactuzumab), PLX3397, AMG820, JNJ-40346527, LY3022855, and others. These monoclonal antibodies can be administered as monotherapy or in combination with other immunotherapy approaches (such as PD-1/PD-L1 blockers and CTLA-4 blockers) to enhance therapeutic outcomes. These monoclonal antibodies are mainly applied to solid tumors, such as lung cancer, colorectal cancer, pancreatic cancer, ovarian cancer, liver cancer, and so on. Currently, these monoclonal antibodies are in phase I or II of clinical trials, and some have been combined with other immune checkpoint inhibitors, demonstrating some safety and efficacy.
The cluster of differentiation 47 or thrombospondin-1 and signal regulatory protein α (CD47/SIRPα) axis is another important signaling pathway that regulates the phagocytosis of macrophages. CD47 is a molecule widely expressed on the surface of normal and tumor cells, which can bind to SIRPα on the surface of macrophages to send a "don't eat me" signal, thereby inhibiting the phagocytosis of tumor cells by macrophages. CD47 is overexpressed in a variety of tumors and is associated with tumor immune escape, therapy resistance, and poor prognosis. Monoclonal antibodies targeting the CD47/SIRPα axis can enhance the phagocytosis of tumor cells by macrophages by blocking the interaction between CD47 and SIRPα, and can also activate the antigen presentation function of macrophages to induce the activation and proliferation of T cells.
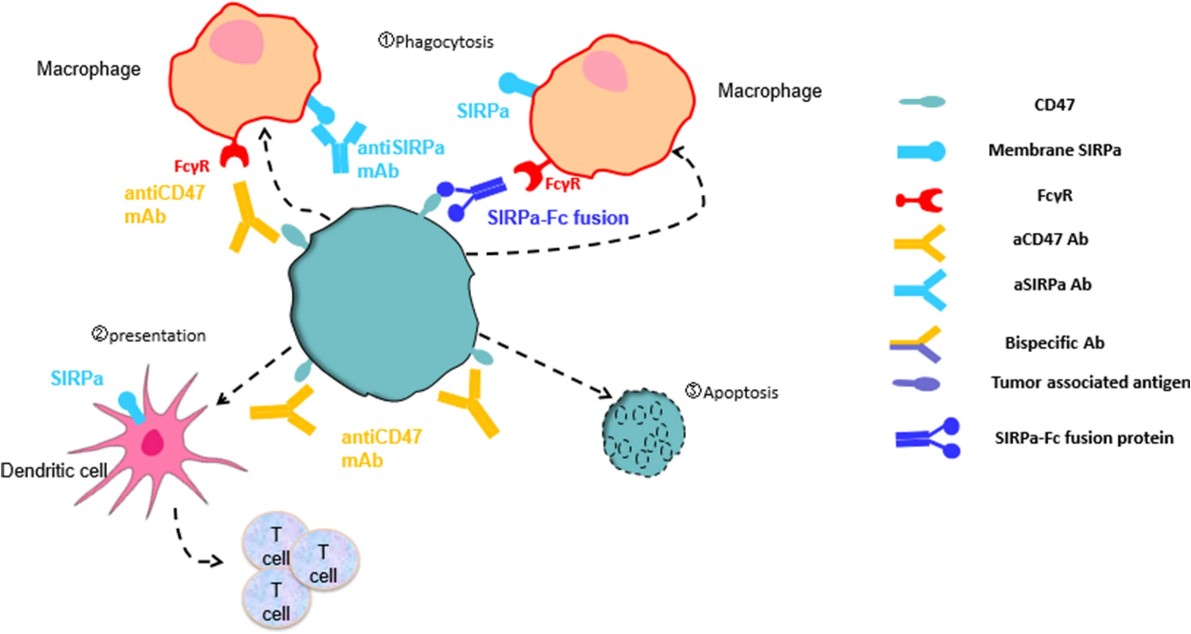 Fig.2 Targeting the CD47/SIRPα Axis in Cancer Immunotherapy (Qu, 2022)
Fig.2 Targeting the CD47/SIRPα Axis in Cancer Immunotherapy (Qu, 2022)
Monoclonal antibodies targeting the CD47/SIRPα axis have reached clinical trials, such as Hu5F9-G4 (Magrolimab), TJC4, CC-90002, ALX148, and others. They can be administered as monotherapy or in combination with other immunotherapy modalities (such as PD-1/PD-L1 inhibitors, CTLA-4 inhibitors, and CD20 antibodies) to augment the antitumor immune response. The main application of these mAbs includes treating hematological neoplasms such as acute myeloid leukemia, chronic lymphocytic leukemia, and non-Hodgkin's lymphoma. Currently, these monoclonal antibodies are in phase I or II of clinical trials, and some have been combined with other immune checkpoint inhibitors or CD20 antibodies, demonstrating some safety and efficacy in terms of overall response rate and overall survival.
The cluster of differentiation 40 (CD40), a cell surface molecule belonging to the TNF receptor superfamily, is mainly expressed on antigen-presenting cells (APCs) such as dendritic cells (DCs), B cells, and macrophages, as well as on some tumor cells. CD40 can activate the antigen presentation and co-stimulatory function of APCs by binding to CD40 ligand (CD40L) on T cells, thereby inducing the activation and proliferation of T cells. In addition, CD40 signaling can also regulate the polarization and phagocytosis of macrophages, as well as B cell differentiation and antibody production. Agonistic monoclonal antibodies targeting CD40 can mimic the effect of CD40L, enhance the immune response of tumor-specific T cells, and also directly inhibit the growth of tumor cells with high CD40 expression.
At present, a variety of monoclonal antibodies targeting CD40 have entered clinical trials, such as CP-870893, RO7009789, APX005M, and SEA-CD40. These monoclonal antibodies can be used not only alone but also in combination with other immunotherapy methods, including PD-1/PD-L1 inhibitors, CTLA-4 inhibitors, and chemotherapy drugs, to achieve better therapeutic effects. These monoclonal antibodies are mainly used to treat solid tumors, such as melanoma, pancreatic cancer, breast cancer, squamous cell carcinoma of the head and neck, etc. Currently, these monoclonal antibodies are in phase I or II of clinical trials, and some have been used in combination with other immune checkpoint inhibitors or chemotherapy drugs, showing certain safety and efficacy.
The C-C motif chemokine ligand 2 or C-C chemokine receptor type 2 (CCL2/CCR2) axis is an important signaling pathway that regulates the migration and polarization of macrophages. CCL2 is a chemokine mainly secreted by tumor cells and inflammatory cells, which can bind to CCR2 on the surface of macrophages and induce the migration of macrophages from bone marrow or surrounding blood into the tumor microenvironment. The CCL2/CCR2 axis is highly expressed in a variety of solid tumors and is associated with tumor development and immune escape. Monoclonal antibodies targeting the CCL2/CCR2 axis can inhibit tumor growth and metastasis by inhibiting the interaction between CCL2 and CCR2, reducing the recruitment and polarization of tumor-associated macrophages (TAMs) to the M2 phenotype.
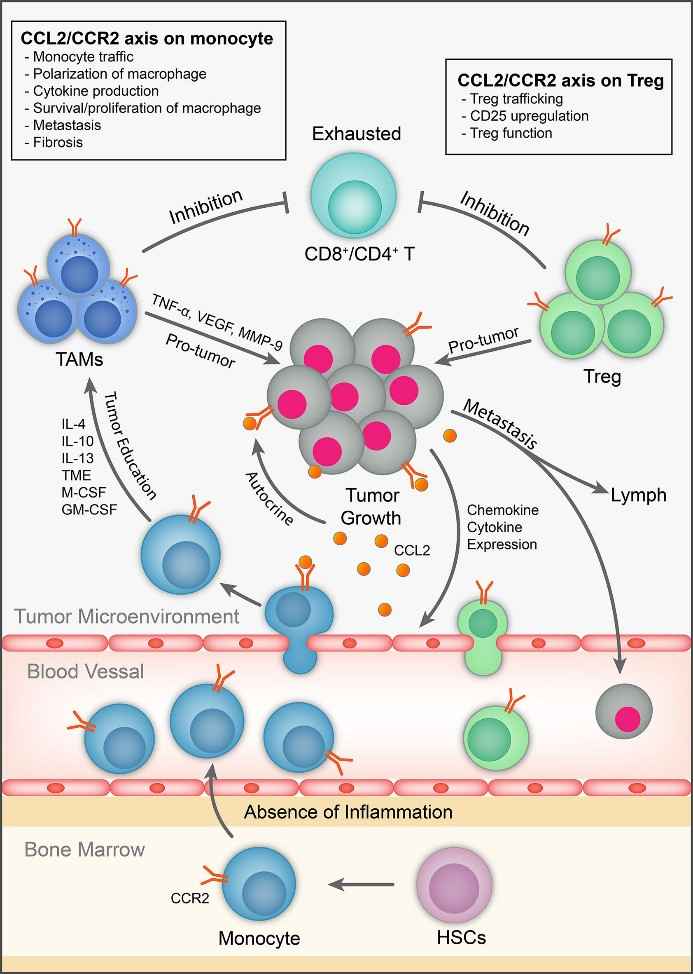 Fig.3 The Role of CCL2/CCR2 Axis in Tumor Immunology. (Fei, 2021)
Fig.3 The Role of CCL2/CCR2 Axis in Tumor Immunology. (Fei, 2021)
Some monoclonal antibodies that block the CCL2/CCR2 pathway have been tested in people, such as Carlumab (CNTO888), PF-04136309, and BMS-813160. These drugs can be used by themselves or with other drugs, such as PD-1/PD-L1 blockers, CTLA-4 blockers, to fight cancer better. These drugs are mainly used for cancers that grow in organs, such as prostate cancer, pancreatic cancer, liver cancer, and others. Right now, these drugs are in early tests in people, and some have been used with other drugs that stop cancer from hiding from the immune system or kill cancer cells.
The C-X-C chemokine receptor type 4 (CXCR4) is a cell surface molecule belonging to the group of G protein-coupled receptors, mainly expressed on macrophages, T cells, B cells, and stem cells, and also expressed on some tumor cells. CXCR4 can regulate cell migration, proliferation, survival, and angiogenesis by binding to stromal-derived factor-1 (SDF-1, also known as CXCL12). CXCR4 is highly expressed in a variety of solid tumors and is associated with tumor invasion, metastasis, and poor prognosis. Monoclonal antibodies targeting CXCR4 can inhibit the growth and spread of tumor cells by blocking the interaction between CXCR4 and CXCL12 and can also affect the recruitment and function of immune cells in the tumor microenvironment.
Monoclonal antibodies targeting CXCR4 have advanced to clinical trials, such as LY2624587, MDX-1338, and BMS-936564. They have the potential to be administered as monotherapy or in combination with other immunotherapy modalities, including PD-1/PD-L1 inhibitors, CTLA-4 inhibitors, and chemotherapy drugs, to enhance the antitumor immune response. These monoclonal antibodies are mainly applied to hematological neoplasms, such as multiple myeloma, acute myeloid leukemia, non-Hodgkin's lymphoma, and others. Notably, they are in phase I or II of clinical trials, and some have been combined with other immune checkpoint inhibitors or chemotherapy drugs, demonstrating some safety and efficacy in terms of overall response rate and overall survival.
Macrophages are a class of highly plastic immune cells that play an important regulatory role in the tumor microenvironment, which can not only promote tumor development and immune escape, but also inhibit tumor growth and metastasis. Monoclonal antibodies targeting macrophages are a novel tumor immunotherapy strategy that can improve the tumor microenvironment, activate tumor-specific T cells, and inhibit tumor cell growth and metastasis through multiple mechanisms. At present, multiple targets and monoclonal antibodies have been discovered and developed, some of which have entered the stage of clinical trials and have shown certain safety and efficacy. However, it is still necessary to further optimize the design and dosage of monoclonal antibodies, explore more reasonable combination therapy schemes, and solve possible problems such as drug resistance and toxic side effects. Monoclonal antibodies targeting macrophages provide a new direction and opportunity for tumor immunotherapy, and are expected to bring better clinical outcomes and quality of life to the majority of cancer patients.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION