Creative Biolabs is a CRO solution company founded and managed by a team of scientists and experts with extensive knowledge and experience in cell therapy research and development. We are dedicated to providing the best products and services to clients worldwide in the academic and industrial sectors, assisting in pursuing breakthrough scientific discoveries to effectively advance preclinical and clinical research.
CD20, also known as B-lymphocyte surface antigen B1, transmembrane 4 structural domain subfamily A member 1 and MS4A1, is a B-lymphocyte surface-specific membrane protein in a non-glycosylated form. CD20 is composed of 297 amino acids and has a relative molecular weight of 33-35 kDa. It consists of four transmembrane domains, one intracellular ring, and two extracellular ring domains, with both the N-terminus and C-terminus located in the cytoplasm. The human CD20 gene is a single copy gene, located on chromosome 11q12.13.1, 16 kp long, with eight exons. Exon I contains the transcription start site, exon III contains the translation start site, exon V is sheared off during splicing, and exon VI encodes the untranslated region at the 3' end of the mRNA of the CD20 molecule. mRNA of the CD20 molecule exists in three forms: the first mRNA is 2.8 Kb long and contains the complete sequence from exon I to exon V. This mRNA is the main form. The second mRNA is 263bp shorter than the former due to the splicing of exon I and exon III, and the third mRNA is 3.4kb long, which is generated by a sequence upstream of exon I that is involved in the splicing within exon I. This form of mRNA is less common.
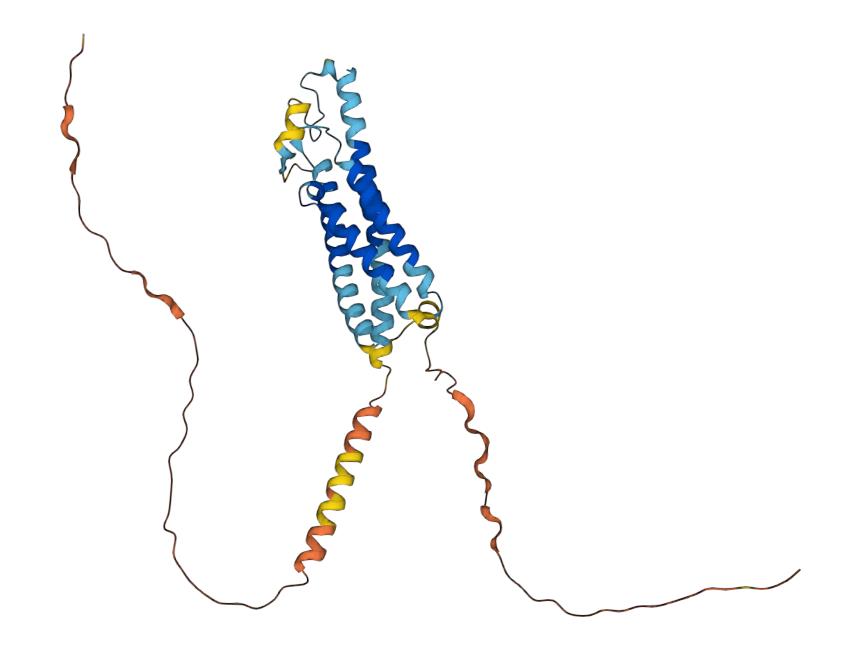 Fig.1 Structure of CD20
Fig.1 Structure of CD20
CD20 is highly expressed on the surface of normal B cells and most malignant B cells. Many studies have shown that it functions as a regulator of calcium channels. Upon binding of the B cell receptor (BCR) to the antigen, CD20 detaches from the BCR complex and forms homo tetramers, which regulate certain calcium ions necessary for antibody response. CD20 is expressed in both early B lymphocytes and mature B lymphocytes, but its expression ceases when they differentiate into plasma cells. CD20 plays an important role in the immune system by triggering intracellular tyrosine kinase signaling, regulating intracellular calcium levels, inducing the accumulation of c-Myc mRNA, and regulating B cell growth and differentiation. The development of immunity requires functional BCR signaling pathways and CD20 has been reported to co-localize in lipid rafts and physically interact directly with the BCR. In addition, it has been observed that mitogen-stimulated CD20 becomes highly phosphorylated and that CD20 functions as a calcium channel and participates in B-cell activation. It has also been shown that cell surface CD20 levels on primary chronic lymphocytic leukemia (CLL) cells correlate with (and may co-regulate) cell surface BCR expression. These studies suggest that CD20 is physiologically directly required for effective BCR signaling in B cells.
CD20 has been expressed in more than 95% of B lymphocytic tumors, and its ease of binding to antibodies that do not readily detach after binding makes the CD20 molecule an ideal target antigen for the treatment of B-cell lymphoma. Moreover, targeting CD20 can also help disrupt the BCR signaling pathway and slow or stop the growth of cancer cells. Chimeric antigen receptor T cell (CAR) therapy can be used to genetically modify a patient's T cells to express a receptor that recognizes and binds to CD20. These modified T cells are then injected into the patient, where they can specifically and accurately recognize CD20 and kill CD20-expressing cancer cells, thereby stopping the growth of cancer cells.
Clinical trials of CAR-T cell therapy targeting CD20 have shown promising results with an overall efficiency of 79% in patients with relapsed or refractory NHL treated with CD19 and CD20 dual-targeted CAR-T therapy. To date, ongoing clinical trials of CD20-targeted CAR therapy have focused on non-Hodgkin's lymphoma (NHL), multiple myeloma (MM), acute lymphoblastic leukemia (ALL), and melanoma. Areas of focus for these trials include CD20/CD19 combination therapies and next-generation CAR design and maintenance therapies. The majority of these ongoing clinical trials are in Phase I, with only a few entering Phase II status. A few of these CD20-targeted CAR cell therapy trials are also being used experimentally in various other immune-related diseases such as immune thrombocytopenic purpura (ITP), systemic lupus erythematosus (SLE), myasthenia gravis (MG), and rheumatoid arthritis (RA).
Table 1. Ongoing CD20-Targeted CAR Cell Therapy Clinical Trials
| NCT Number | Title | Status | Conditions | Sponsor/Collaborators | Phases |
| NCT04007029 | Modified Immune Cells (CD19/CD20 CAR-T Cells) in Treating Patients With Recurrent or Refractory B-Cell Lymphoma or Chronic Lymphocytic Leukemia | Recruiting | CD19 Positive|CD20 Positive|Recurrent Chronic Lymphocytic Leukemia|Recurrent Diffuse Large B-Cell Lymphoma|Recurrent Follicular Lymphoma|Recurrent Mantle Cell Lymphoma|Recurrent Primary Mediastinal (Thymic) Large B-Cell Cell Lymphoma|Recurrent Small Lymphocytic Lymphoma|Refractory Chronic Lymphocytic Leukemia|Refractory Diffuse Large B-Cell Lymphoma|Refractory Follicular Lymphoma|Refractory Mantle Cell Lymphoma|Refractory Primary Mediastinal (Thymic) Large B-Cell Cell Lymphoma|Refractory Small Lymphocytic Lymphoma | Jonsson Comprehensive Cancer Center|Parker Institute for Cancer Immunotherapy | Phase 1 |
| NCT04697940 | Decitabine-primed Tandem CD19/CD20 CAR-T Cells Treatment in r/r B-NHL | Recruiting | Relapsed and Refractory B-cell Non-Hodgkin's Lymphoma|Decitabine-primed Tandem CD19/CD20 CAR-T Cells | Han weidong|Chinese PLA General Hospital | Phase 1|Phase 2 |
| NCT04735471 | A Safety and Efficacy Study of ADI-001, an Anti-CD20 Allogeneic Gamma Delta CAR-T, in Subjects With B Cell Malignancies | Recruiting | Lymphoma, Follicular|Lymphoma, Mantle-Cell|Marginal Zone Lymphoma|Primary Mediastinal B-cell Lymphoma|Diffuse Large B Cell Lymphoma|Lymphoma, Non-Hodgkin | Adicet Bio, Inc | Phase 1 |
| NCT04430530 | 4SCAR-T Therapy Post CD19-targeted Immunotherapy | Recruiting | CD19 Negative B-cell Malignancies | Shenzhen Geno-Immune Medical Institute|ShiJiaZhuang Zhongxi Children Hospital|Shenzhen Children's Hospital|The Seventh Affiliated Hospital of Sun Yat-sen University | Phase 1|Phase 2 |
| NCT04186520 | CAR-20/19-T Cells in Patients With Relapsed Refractory B Cell Malignancies | Recruiting | Non Hodgkin Lymphoma (NHL)|Mantle Cell Lymphoma (MCL)|Chronic Lymphocytic Leukemia (CLL)|Follicular Lymphoma|Marginal Zone Lymphoma|Diffuse Large B Cell Lymphoma|Primary Mediastinal Large B-cell Lymphoma (PMBCL) | Medical College of Wisconsin | Phase 1|Phase 2 |
| NCT04989803 | Study Evaluating the Safety and Efficacy of KITE-363 in Participants With Relapsed and/or Refractory B-cell Lymphoma | Recruiting | Relapsed and/or Refractory B-cell Lymphoma | Kite, A Gilead Company|Gilead Sciences | Phase 1 |
| NCT03664635 | MB-CART20.1 Lymphoma | Active, not recruiting | Relapsed or Refractory B-cell Non-Hodgkin's Lymphoma|Non-Hodgkin's Lymphoma|B-cell Lymphoma Refractory|B-cell Lymphoma Recurrent | Miltenyi Biomedicine GmbH | Phase 1|Phase 2 |
| NCT03893019 | MB-CART20.1 Melanoma | Recruiting | Melanoma (Skin) | Miltenyi Biomedicine GmbH|DLR German Aerospace Center | Early Phase 1 |
| NCT05618041 | The Safety and Efficacy Investigation of CAR-T Cell Therapy for Patients With Hematological Malignancies | Recruiting | Acute Lymphoblastic Leukemia|Lymphoma|Multiple Myeloma | Hebei Senlang Biotechnology Inc., Ltd. | Not Applicable |
| NCT03277729 | A Phase I/II Study to Evaluate the Safety of Cellular Immunotherapy Using Autologous T Cells Engineered to Express a CD20-Specific Chimeric Antigen Receptor for Patients With Relapsed or Refractory B Cell Non-Hodgkin Lymphomas | Recruiting | Recurrent B-Cell Non-Hodgkin Lymphoma|Recurrent Chronic Lymphocytic Leukemia|Recurrent Diffuse Large B-Cell Lymphoma|Recurrent Follicular Lymphoma|Recurrent Lymphoplasmacytic Lymphoma|Recurrent Mantle Cell Lymphoma|Recurrent Marginal Zone Lymphoma|Refractory B-Cell Non-Hodgkin Lymphoma|Refractory Diffuse Large B-Cell Lymphoma|Refractory Follicular Lymphoma|Refractory Lymphoplasmacytic Lymphoma|Refractory Mantle Cell Lymphoma|Refractory Transformed Non-Hodgkin Lymphoma|Recurrent Transformed B-Cell Non-Hodgkin Lymphoma|Recurrent Transformed Chronic Lymphocytic Leukemia|Refractory Marginal Zone Lymphoma|Refractory Transformed B-Cell Non-Hodgkin Lymphoma|Refractory Transformed Chronic Lymphocytic Leukemia|Recurrent Small Lymphocytic Lymphoma|Refractory Chronic Lymphocytic Leukemia|Refractory Small Lymphocytic Lymphoma|Recurrent Central Nervous System Lymphoma|Refractory Central Nervous System Lymphoma | Fred Hutchinson Cancer Center|Mustang Bio | Phase 1|Phase 2 |
| NCT04283006 | A Study of CD20/CD22 Targeted CAR T-cell Therapy for Relapsed or Refractory Lymphoid Malignancies | Recruiting | Relapsed and Refractory|Lymphoid Hematological Malignancies | He Huang|Yake Biotechnology Ltd.|Zhejiang University | Early Phase 1 |
| NCT05421663 | A Study of C-CAR039 in Patients With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma | Recruiting | B Cell Non-Hodgkin's Lymphoma | Cellular Biomedicine Group, Inc.|City of Hope Medical Center | Phase 1 |
| NCT05418088 | Genetically Engineered Cells (Anti-CD19/CD20/CD22 CAR T-cells) for the Treatment of Relapsed or Refractory Lymphoid Malignancies | Recruiting | Recurrent Acute Lymphoblastic Leukemia|Recurrent B Acute Lymphoblastic Leukemia|Recurrent B-Cell Prolymphocytic Leukemia|Recurrent Chronic Lymphocytic Leukemia|Recurrent High Grade B-Cell Lymphoma|Recurrent Indolent Non-Hodgkin Lymphoma|Recurrent Non-Hodgkin Lymphoma|Recurrent Transformed Chronic Lymphocytic Leukemia|Refractory Acute Lymphoblastic Leukemia|Refractory B Acute Lymphoblastic Leukemia|Refractory B-Cell Prolymphocytic Leukemia|Refractory Chronic Lymphocytic Leukemia|Refractory High Grade B-Cell Lymphoma|Refractory Indolent Non-Hodgkin Lymphoma|Refractory Non-Hodgkin Lymphoma|Refractory Transformed Chronic Lymphocytic Leukemia | Sumithira Vasu|Ohio State University Comprehensive Cancer Center | Phase 1 |
| NCT05149391 | A Study of C-CAR039 in Subjects With Relapsed and/or Refractory B Cell Non-Hodgkin's Lymphoma | Recruiting | B Cell Non-Hodgkin's Lymphoma | Peking University|Peking University Cancer Hospital & Institute | Phase 1 |
| NCT04215016 | Safety of Autologous Humanized Anti-CD19 and Anti-CD20 Dual Specific CAR-T Cells in Adult Patients With Diffuse Large B-cell Lymphoma | Recruiting | Relapsed or Refractory DLBCL Patients With Either CD19 or CD20 Positive | Fujian Medical University | Phase 1 |
| NCT04049383 | CAR-20/19-T Cells in Pediatric and Young Adult Patients With Relapsed/Refractory B Cell ALL | Recruiting | Acute Lymphoblastic Leukemia, in Relapse|Acute Lymphoblastic Leukemia With Failed Remission|Acute Lymphoblastic Leukemia Recurrent|Acute Lymphoblastic Leukemia Not Having Achieved Remission|Acute Lymphoblastic Leukemia, Pediatric|Acute Lymphoblastic Leukemia | Medical College of Wisconsin|Children's Hospital and Health System Foundation, Wisconsin | Phase 1 |
| NCT04429438 | Multi-CAR-T Cells Targeting B Cell Lymphomas | Recruiting | B Cell Lymphoma (BCL) | Shenzhen Geno-Immune Medical Institute|The Seventh Affiliated Hospital of Sun Yat-sen University|Shenzhen Children's Hospital | Phase 1|Phase 2 |
| NCT04889716 | CAR-T Followed by Bispecific Antibodies | Recruiting | Large B-cell Lymphoma | Abramson Cancer Center of the University of Pennsylvania|Genentech, Inc. | Phase 2 |
| NCT04016129 | CAR-T Immunotherapy Targeting CD19- ALL | Recruiting | B-cell Leukemia | Shenzhen Geno-Immune Medical Institute | Phase 1|Phase 2 |
| NCT05388695 | To Observe the Dual-target Chimeric Antigen Receptor T Cells in the Treatment of B Cell Hematologic Tumors | Recruiting | 19 and 22+ B Cell Hematologic Tumors|19 and 20+ B Cell Hematologic Tumors | Hebei Senlang Biotechnology Inc., Ltd.|Tongji Hospital | Phase 1 |
| NCT03407859 | Sequential Treatment With CD20/CD22/CD10-CART After CD19-CART Treatment Base on MRD in Relapsed/Refractory B-ALL | Recruiting | Therapy Related Leukemia | Zhujiang Hospital|Nanfang Hospital of Southern Medical University | Early Phase 1 |
| NCT05010564 | CAR-T Cell, B-cell Acute Lymphoblastic Leukemia (TriCAR) | Not yet recruiting | Leukemia, B-Cell | Baylor College of Medicine|Texas Children's Cancer Center | Phase 1 |
| NCT05094206 | CAR20.19.22 T-cells in Relapsed, Refractory B-cell Malignancies | Recruiting | B-cell Non Hodgkin Lymphoma|B-cell Chronic Lymphocytic Leukemia | Medical College of Wisconsin|Miltenyi Biomedicine GmbH | Phase 1 |
| NCT04792489 | DALY 2.0 USA/ MB-CART2019.1 for DLBCL | Recruiting | DLBCL | Miltenyi Biomedicine GmbH | Phase 2 |
| NCT03870945 | Safety of MB-CART2019.1 in Lymphoma Patients (MB-CART2019.1 Lymphoma / DALY 1) | Active, not recruiting | B-cell Non Hodgkin Lymphoma | Miltenyi Biomedicine GmbH|ICON plc | Phase 1|Phase 2 |
| NCT05292898 | A Triple-targeted Cell Preparation Targeting CD19/CD20/CD22 in Patients With Relapsed/Refractory B-cell Acute Lymphocytic Leukemia | Recruiting | Acute Lymphocytic Leukemia | Institute of Hematology & Blood Diseases Hospital|Nanjing Legend Biotech Co. | Phase 1 |
| NCT04074330 | A Study of TAK-981 Given With Rituximab in Adults With Relapsed or Refractory CD20-Positive Non-Hodgkin Lymphoma | Active, not recruiting | Lymphoma, Non-Hodgkin | Takeda | Phase 1|Phase 2 |
| NCT05169489 | A Study of bbT369 in Relapsed and/or Refractory B Cell Non-Hodgkin's Lymphoma (NHL) | Recruiting | Diffuse Large B Cell Lymphoma (DLBCL) | 2seventy bio | Phase 1|Phase 2 |
| NCT04844866 | Efficacy and Safety of MB-CART2019.1 vs. SoC in Lymphoma Patients | Recruiting | Diffuse Large B-cell Lymphoma | Miltenyi Biomedicine GmbH|ICON plc | Phase 2 |
| NCT05318963 | Targeting CD19/CD20/CD22 Triple-targeted Cell in Patients With Relapsed/Refractory B-cell Lymphoma | Recruiting | B-cell Lymphoma Recurrent|B-cell Lymphoma Refractory | Qiu Lugui|Nanjing Legend Biotech Co.|Institute of Hematology & Blood Diseases Hospital | Phase 1 |
| NCT05379647 | Natural Killer (NK) Cell Therapy for B-Cell Malignancies | Recruiting | B-cell Lymphoma|B-cell Acute Lymphoblastic Leukemia | Zhejiang University|Hangzhou Qihan Biotech Co., Ltd. | Phase 1 |
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION