Bispecific T cell engager is a class of antibody molecules with two different antigen-binding sites, which can simultaneously bind to tumor-associated antigen (TAA) on tumor cells and CD3 molecules on T cells, thereby binding T Cell redirection to the surface of tumor cells activates T cell proliferation and cytotoxicity to kill tumor cells. Bispecific T-cell engagers are a specialized format of bispecific antibodies (BsAbs) that consist of two single-chain variable fragments (scFv), one scFv that binds CD3 and the other scFv that binds TAA. Bispecific T-cell engagers have a molecular weight of approximately 55 kDa, much smaller than conventional IgG antibodies (approximately 150 kDa).
The mechanism of action of bispecific T-cell engager is not complicated. First, by binding to CD3, bispecific T-cell engagers activate T cells and induce their expression of effector molecules, such as perforin and granzymes. Bispecific T-cell engagers then recruit T cells to the vicinity of tumor cells and promote the formation of immune synapses by binding to TAA. Finally, T cells release effector molecules that are cytotoxic to tumor cells and induce apoptosis. The course of action of bispecific T-cell engager does not depend on the specificity of the T cell receptor (TCR) or the restriction of the major histocompatibility complex (MHC).
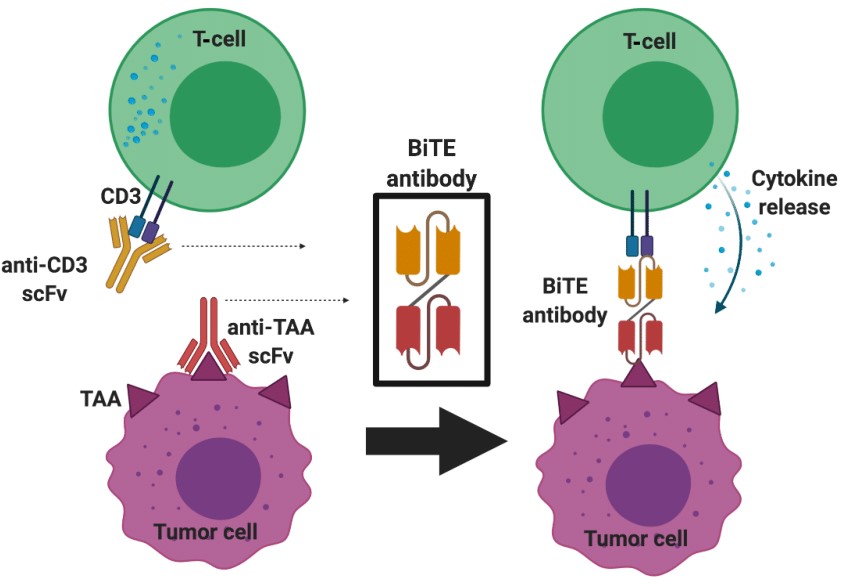 Fig.1 Schematic illustration of Bispecific T-cell Engager) structure and mechanism of action (Rallis, 2021)
Fig.1 Schematic illustration of Bispecific T-cell Engager) structure and mechanism of action (Rallis, 2021)
Bispecific T-cell engagers have multiple advantages in tumor immunotherapy. First, T cells widely present in the human body can be used as effectors without exogenous expansion or modification; Second, bispecific T-cell engagers can target various types of tumor-associated antigens, including intracellular antigens, lineage antigens and mutant antigens; Third, bispecific T-cell engagers can Effectively infiltrate solid tumors and play a role in the tumor microenvironment; Fourth, bispecific T-cell engagers can avoid the suppression of immune checkpoints or the occurrence of drug resistance.
The development of bispecific T-cell engagers can be traced back to the 1980s, when scientists began to try to prepare antibody molecules with two different antigen-binding sites to achieve multiple functions. The earliest BsAbs were prepared by chemically cross-linking two monoclonal antibodies or their fragments, but this method has many disadvantages, such as low yield, high heterogeneity, low stability and high immunogenicity. With the advancement of molecular biology and protein engineering technology, people have developed a variety of methods based on recombinant DNA technology to prepare BsAb, such as four-chain BsAb, double-chain BsAb, single-chain BsAb and so on. Among them, the single-chain BsAb is composed of two single-chain variable fragments (scFv) connected by a linker, which has the advantages of smaller molecular weight, higher tissue permeability and lower immunogenicity.
Bispecific T-cell engager was first developed by Micromet in Germany, and its representative product is blinatumomab, a bispecific T-cell engager molecule targeting CD19 and CD3. Blinatumomab was approved by the FDA in 2014 for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL), becoming the first marketed bispecific T-cell engager molecule. Since then, blinatumomab has been approved by the FDA for the treatment of other types of B-cell malignancies, such as non-Hodgkin's lymphoma (NHL) and large B-cell lymphoma (DLBCL).
As a new type of tumor immunotherapy, bispecific T-cell engager has made remarkable progress and achievements in the past few decades. However, bispecific T-cell engager still faces some challenges and limitations, such as short half-life, high dose, cytokine release syndrome (CRS) management are required. To overcome these problems, people are developing a new generation of bispecific T-cell engager molecules, such as increasing the Fc domain to prolong the half-life, adding a safety switch to control dose and toxicity, adding a conditional activation mechanism to improve tumor specificity, etc. The development prospect of bispecific T-cell engager is still broad, and it is expected to bring new treatment options and hope to more types of tumor patients.
The clinical progress of bispecific T cell engager mainly involves two fields of hematological malignancies and solid tumors. Currently, one bispecific T-cell engager molecule has been approved by the FDA, blinatumomab, for the treatment of B-cell acute lymphoblastic leukemia (B-ALL) and other B-cell malignancies. In addition, there are multiple bispecific T-cell engager molecules in clinical trials at different stages, targeting different tumor-associated antigens, such as EpCAM, CEA, PSMA, HER2, EGFR, etc.
In the field of solid tumors, a recent major breakthrough was tebentafusp, a bispecific T-cell engager molecule targeting gp100 and CD3. tebentafusp received FDA approval in January 2021 for the treatment of HLA-A02:01-positive unresectable or metastatic uveal melanoma (mUM), becoming the first bispecific T-cell engager molecule approved for a solid tumor and the first An innovative drug for the treatment of mUM. The approval of tebentafusp is based on positive data from the IMCgp100-202 clinical trial, which enrolled 378 HLA-A02:01-positive mUM patients and randomly assigned them to receive tebentafusp or standard treatment regimens such as pembrolizumab, ipilimumab, or dacarbazine. The results showed that the median overall survival in the tebentafusp group was 21.7 months, which was significantly higher than the 16.0 months in the standard treatment group (hazard ratio 0.68, 95% confidence interval 0.53-0.87, P=0.003).
In addition to tebentafusp, there are other bispecific T-cell engager molecules in clinical trials in solid tumors, such as AMG 110 for epithelial cell adhesion molecule (EpCAM) and CD3, MEDI-565 for carcinoembryonic antigen (CEA) and CD3, AMG 160 for prostate-specific membrane antigen (PSMA) and CD3, ZW25 for human epidermal growth factor receptor 2 (HER2) and CD3, etc. These bispecific T-cell engager molecules are expected to bring new treatment options and hope to patients with solid tumors.
Although bispecific T cell engager has shown great potential in tumor immunotherapy, it still faces some challenges and limitations, mainly including the following aspects:
In response to the above challenges and limitations, people are developing a new generation of bispecific T-cell engager molecules or adopting corresponding improvement strategies, mainly including the following aspects:
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION