Natural killer (NK) cells were the first subtype of innate lymphoid cells (ILCs) to be identified and can respond to virally infected and/or transformed cells with a variety of effector functions, chiefly cell killing and production of pro-inflammatory cytokines. NK cells play a crucial role in triggering the antitumor immune response. Despite their activity in controlling tumor growth, NK cells are susceptible to multiple immunosuppressive mechanisms that are active in the TME. The field of NK cell-based immunotherapy of cancer has reached an exciting juncture.
Impaired NK cell function has been well documented in both solid tumors and hematologic malignancies. Many factors can lead to dysfunctional NK cells in cancer. The TME is a major obstacle to ensuring the optimum antitumor activity of NK cells, in which immunosuppressive cells and molecules limit NK cell activity. Accumulating evidence indicates that TME produced soluble modulators that negatively regulate the maturation, proliferation, and effector function of NK cells.
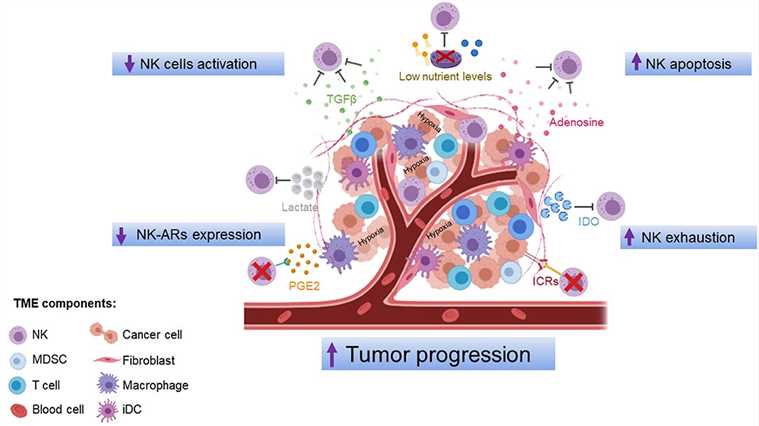 Fig.1 Mechanisms of NK cell dysfunction in the TME.1,3
Fig.1 Mechanisms of NK cell dysfunction in the TME.1,3
Tumor cells themselves may affect the ability of NK cells to infiltrate the tumor mass via several mechanisms. Cells from the TME may also have suppressive activities against NK cells. Thus, efforts should be made to improve NK cell recruitment and activation in the tumor bed.
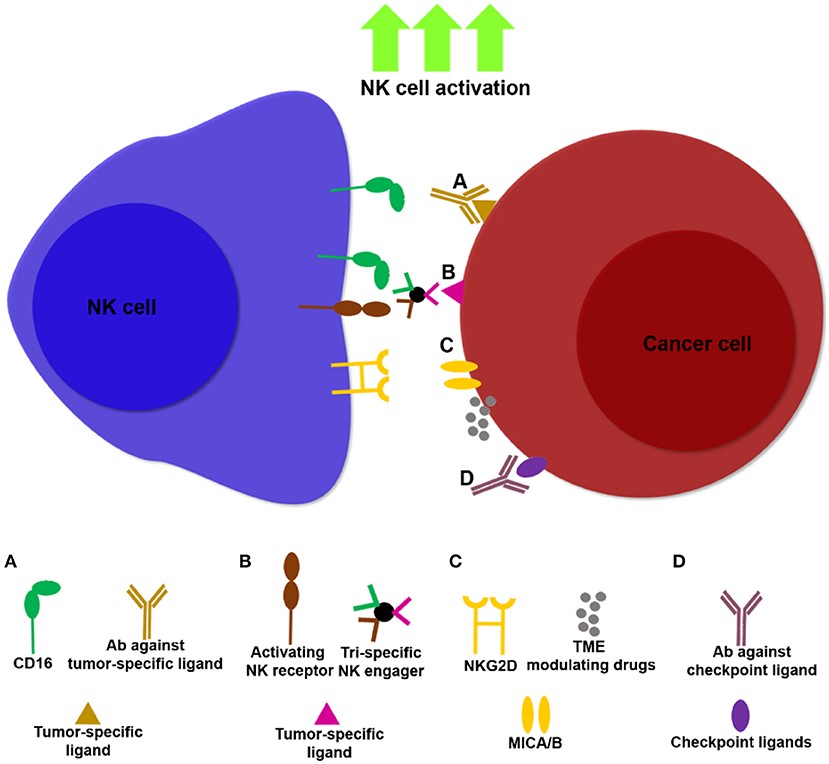 Fig.2 Examples of NK cell immunotherapies targeting NK cells and tumors.2,3
Fig.2 Examples of NK cell immunotherapies targeting NK cells and tumors.2,3
NK cell adoptive cellular immunotherapy can provide a large number of activated NK cells and exert the antitumor effect.
The study of the TME has completely transformed our cancer cell-centric view of tumor biology. Many strategies focusing on improving NK cells have been attempted to reshape TME. As a leading cell therapeutics biotech that provides cell therapy-related services, Creative Biolabs has developed a series of novel platforms for enhanced CAR-NK Therapy. We provide one-stop CAR-NK therapy development services, Custom iPSC-derived NK Cell Service to satisfy your demands. If you have specific research questions or requirements, please feel free to contact us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION