The T cell receptor (TCR) is a highly intricate receptor within the body. It is composed of an octameric signaling complex that triggers a tightly regulated signaling cascade in T cells, controlling various cell functions such as migration, cytokine release, cytotoxicity, survival, proliferation, and differentiation. Although the contribution and interplay of its six different receptor subunits to its broad signaling activities are just beginning to emerge, it is known that the TCR is composed of one TCRα and one TCRβ chain, which bind to the peptide-MHC ligand, and three signaling subunits collectively called CD3: one dimer of CD3ε with CD3γ, one dimer of CD3ε with CD3δ, and one CD3ζ homodimer. All subunits are type I membrane proteins, and all but CD3ζ have extracellular immunoglobulin (Ig) domains.
T cell receptor fusion construct (TRuC) T cells are designed to harness all signaling subunits of the TCR to activate T cells independently of the peptide-major histocompatibility complex (MHC), usually the human leukocyte antigen (HLA). TRuC-T cells are endowed with a fusion protein that comprises a tumor-antigen binder tethered to full-length CD3ε or γ subunits. This TRuC is assembled into the TCR and reprograms T cells to kill tumor cells expressing the cognate surface antigen, much like natural CD3 subunits.
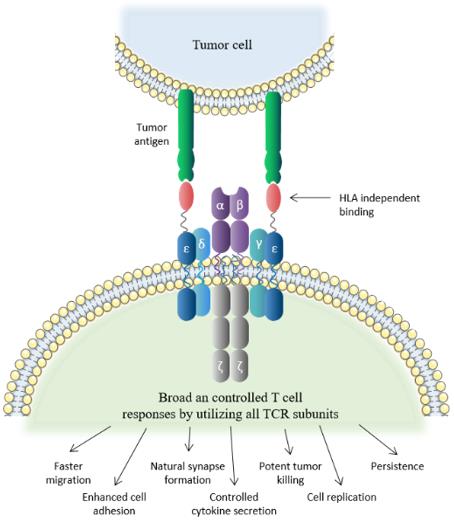 Fig 1 TRuC-T cell
Fig 1 TRuC-T cell
One of the most potent therapeutic strategies in immunotherapy is adoptive cell therapy (ACT), which involves the use of T cells with engineered receptors. Chimeric antigen receptors (CAR) and engineered TCRs are the two most recent and effective types of manufactured receptors used in adoptive T-cell immunotherapy. CAR-T cells consist of three main components: an extracellular region, a transmembrane region, and an intracellular signaling domain. The extracellular region is typically a single-chain antibody (scFv) that recognizes and binds to the target antigen, while the transmembrane region anchors the scFv to the cell membrane. The intracellular signaling domain consists of co-stimulatory factors and CD3 signaling domains, which activate T cells and induce effector functions.
On the other hand, TCR-engineered T cells express tumor antigen-specific receptors with α and β chains, which are produced from high-quality and high-avidity antigen-specific T-cell clones. These cells are utilized to develop antigen-specific immunotherapy, and their high affinity and cellular avidity determine the specific binding of several cellular proteins, playing a crucial role in activating T cellular avidity. While the low affinity of TCRs reduces the efficiency of TCR-based therapy, high-affinity TCRs (Affinity ≥ 2.5nM) are specific and sensitive for targeting cell-surface HLA.
In ACT, cells are collected from cancer tissues/T cells, isolated from other cells, genetically manipulated by engineered TCR or CAR, co-cultured, proliferated, and then reintroduced into circulation. This process results in the generation of T cells with enhanced tumor targeting and killing abilities, making them a promising therapy for cancer treatment.
However, current T-cell therapy approaches have limitations and side effects. For instance, CAR-T cell therapy commonly leads to cytokine release syndrome (CRS), where the newly transferred T cells, or other immune cells responding to the transferred T cells, release excessive cytokines into the bloodstream. Additionally, TCR-T cell therapy can cure solid tumors and hematology but is limited by HLA matching. TRuCs, on the other hand, are antibody-based binding domains fused to TCR subunits, designed to recognize tumor surface antigens effectively. TRuCs consist of specific ligand antibodies fused to the extracellular N-termini of five TCR subunits (TCRα, TCRβ, CD3ϵ, CD3γ, and CD3δ), offering engineered T cells new target specificity and HLA-independent target cell elimination ability, which can be activated by corresponding target cells. Furthermore, upon lentiviral transduction, the TRuCs are integrated into the native TCR complex on the T cell surface, thereby retaining the activation and effector function of T cells. Importantly, unlike CAR-T cell therapy, TRuC-T cells can be controlled in terms of cytokine release, greatly minimizing the risk of CRS.
In terms of the treatment potential for blood tumors, TRuC-T cells may be even more effective than CAR-T cells. Although CD19-specific CAR-T cells have demonstrated impressive therapeutic and curative potential in treating B cell malignancies, with complete response rates up to 90% reported for pediatric and adult patients with relapsed or refractory acute B-lymphoblastic leukemia (ALL), and around 50% for relapsed or refractory non-Hodgkin's lymphoma (NHL) patients, current T cell therapy approaches have some limitations.
One of the most common side effects of CAR-T cell therapy is cytokine release syndrome (CRS), caused when transferred T cells or other immune cells responding to the new T cells release a large number of cytokines into the blood. In contrast, CD19-targeted CAR-T cells have been shown to potently eliminate tumors in mouse models while releasing fewer cytokines than existing CD19-targeted CAR-T cells, without the need for an additional co-stimulatory domain. In a mouse xenograft model using Raji cells, CD3ε-based TRuC-T cells showed better anti-tumor activity than 28ζ and BBζ CARs. The enhanced in vivo activity of TRuC-T over CAR-T cells relates to profound differences in T cell signaling.
Although adoptive cell therapy with CAR-T cells has shown unprecedented clinical efficacy against hematological malignancies, monotherapy with CAR-T cells has suboptimal clinical efficacy against solid tumors. One primary underlying factor preventing CAR-T cell success in solid tumor indications is the failure to initiate and elicit a complete TCR response. In contrast, TRuC-T cells better leverage native TCR signaling and employ the full signaling machinery of the TCR complex, as opposed to CARs that use the limited signaling capacity of an isolated CD3ζ cytoplasmic tail. Therefore, TRuC-T cells may be a promising alternative to address the suboptimal efficacy of existing CAR-T therapies for solid tumors.
Preclinical evidence supports the efficacy of TRuC-T cells reprogrammed to target mesothelin, a solid tumor antigen. While mesothelin is strongly expressed by solid tumor cells, it is weakly expressed by normal mesothelial cells. CAR-T cell therapy that uses the CD3ζ chain fused to one or more co-stimulatory domains may be limited in efficacy in solid tumors due to insufficient or aberrant signaling. Additionally, extrinsic immunosuppressive factors in the solid tumor microenvironment (TME) may limit the accumulation and proliferation of CAR-T cells. However, MSLN-targeted TRuC-T cells, TC-210 T cells, have demonstrated potent in vitro activity and can eliminate MSLN-expressing tumors in mice. Reports show that TC-210 T cells effectively kill MSLN+ tumor cells in vitro and efficiently eradicate MSLN+ mesothelioma, lung, and ovarian cancers in xenograft mouse tumor models. TC-210 T cells show overall comparable efficacy to MSLN-targeted BBζ CAR-T cells but consistently exhibit faster tumor rejection kinetics due to earlier intratumoral accumulation and earlier signs of activation. In vitro and ex vivo metabolic profiling suggests that TC-210 T cells have lower glycolytic activity and higher mitochondrial metabolism than MSLN-BBζ CAR-T cells. In summary, TC-210 T cells show promise as a cell therapy for treating cancers expressing MSLN, and their differentiated features from CAR-T cells may lead to improved effects and safety in treating solid tumors.
It has been developed a pipeline of novel T-cell therapies for patients suffering from solid tumors or hematological malignancies. The aim is to combine the best features of CAR-T therapy and conventional TCR-based therapy, while overcoming their associated limitations, by using the complete, regulated TCR complex and allowing it to be HLA-independent. The TRuC constructs have been designed to recognize highly expressed surface antigens on tumor cells, activating the "complete TCR machinery," including all subunits, to "generate a broad and controlled response" which has been shown in preclinical studies to translate into longer-lasting responses with potentially fewer adverse events. These characteristics are expected to enable the expansion of indications to ones that were "previously untreatable by earlier cell therapies due to adverse events" such as CRS or an inability to withstand the complex, immunosuppressive microenvironment surrounding solid tumors. The TRuC-T cell product candidate targeting solid tumors, gavo-cel, has been studied in a Phase 1/2 clinical trial to treat patients with mesothelin-positive non-small cell lung cancer (NSCLC), ovarian cancer, malignant pleural/peritoneal mesothelioma, and cholangiocarcinoma. In preclinical studies, the efficacy of TC-210 was compared to the efficacy of MSLN-specific CARs (MSLN-CARs) in mouse models of mesothelioma, NSCLC, and ovarian carcinoma. According to reports, results showed that TC-210 exhibited potent in vivo activity in mesothelioma, NSCLC, and ovarian carcinoma models. TC-210 showed faster migration to the tumor, faster kill kinetics, lower cytokine release compared to CAR-T cells, and functional persistence in a mesothelioma re-challenge model.
Furthermore, the TRuC-T cell product candidate targeting hematological malignancies, TC-110, has been studied in a Phase 1/2 clinical trial to treat patients with CD19-positive adult acute lymphoblastic leukemia (aALL) and aggressive or indolent non-Hodgkin lymphoma (NHL). In preclinical studies, the efficacy of TC-110 was compared to the efficacy of CD19-specific CARs (CD19-CARs) in mouse models of acute lymphoblastic leukemia and Burkitt's lymphoma. According to reports, the results showed that TC-110 demonstrated potent in vivo efficacy in xenogeneic models of both diseases. Tumor-bearing mice showed faster tumor regression after treatment with TC-110 compared to treatment with CD19 CARs (for both diseases). In addition, cytokine release declined rapidly after TC-110 treatment and correlated with the time of active tumor regression.
TRuC-T cell-based therapies are expected to offer similar efficacy with reduced adverse events compared to CAR-T cell technology. Thus, TRuC-T cell therapy has shown promising preclinical and clinical results in the gavo-cel clinical trial.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION