A chimeric antigen receptor (CAR) is a synthetic fusion protein that enables T cells to recognize and eliminate specific target cells. The concept emerged from the pioneering work of Zelig Eshhar and colleagues in the late 1980s, who first demonstrated that T cells could be genetically modified to express chimeric receptors capable of antibody-type specificity.
Unlike conventional antibody treatments, these engineered receptors combine the specificity of monoclonal antibodies with the cytotoxic potential of T cells, creating a hybrid molecular structure. The innovation stems from the recognition that while T cells are highly proficient at eliminating threats, they often struggle to identify cancer cells that have evolved sophisticated evasion mechanisms. A critical advantage of CAR-modified T cells is their ability to recognize native cell surface antigens without the need for antigen processing and presentation. This direct recognition enables targeting of a broader range of molecular structures, including carbohydrates and glycolipids, which are typically invisible to conventional T cells.
The development of chimeric antigen receptor technology reached a pivotal moment with Carl June's breakthroughs at the University of Pennsylvania. June's team achieved remarkable success in treating chronic lymphocytic leukemia patients with CD19 CAR-T cells, demonstrating unprecedented response rates and establishing CAR-T cell therapy as a viable treatment option. The CAR-T therapy process involves collecting a patient's T cells through leukapheresis, genetically engineering them to express chimeric antigen receptors that target specific cancer antigens, expanding the modified cells in the laboratory, and then reinfusing them back into the patient after lymphodepletion. Once in the body, these engineered T cells can recognize and bind to cancer cells through their CAR receptors, triggering T cell activation, proliferation, and cytotoxic responses that effectively eliminate cancer cells while also establishing a population of memory cells for long-term surveillance.
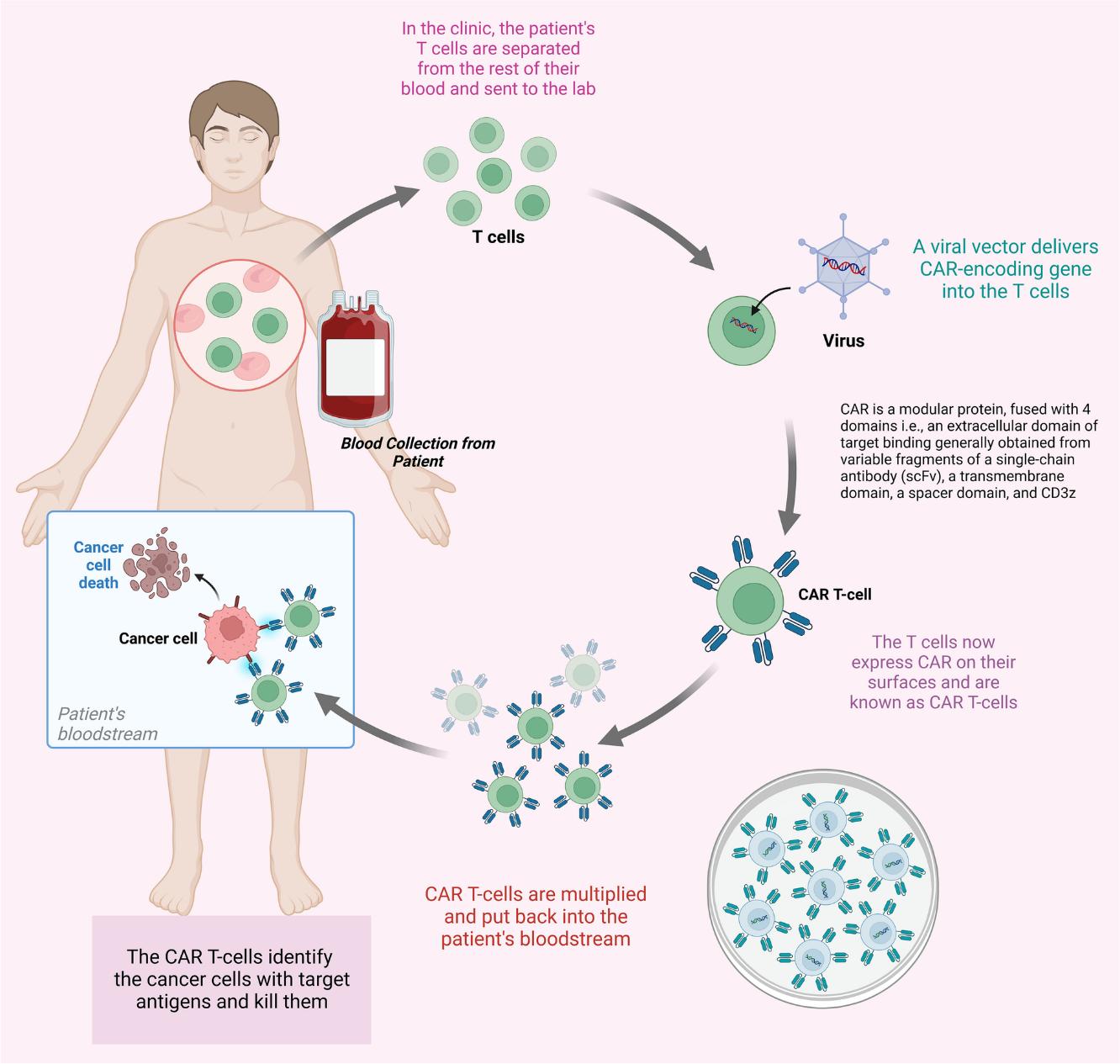 Fig.1 Process of CAR T-cell therapy1,4.
Fig.1 Process of CAR T-cell therapy1,4.
The chimeric antigen receptor discovery has revolutionized cancer treatment, particularly in the field of immunotherapy. CAR-T cells can potentially target any surface-expressed molecule, regardless of its biological function, expanding the repertoire of potential cancer antigens and opening new possibilities for treating various types of cancer.
The structure of chimeric antigen receptor follows a modular design principle, with each component carefully engineered to serve specific functions. At its core, the receptor comprises four main domains, each playing a crucial role in the overall functionality of the engineered T cell.
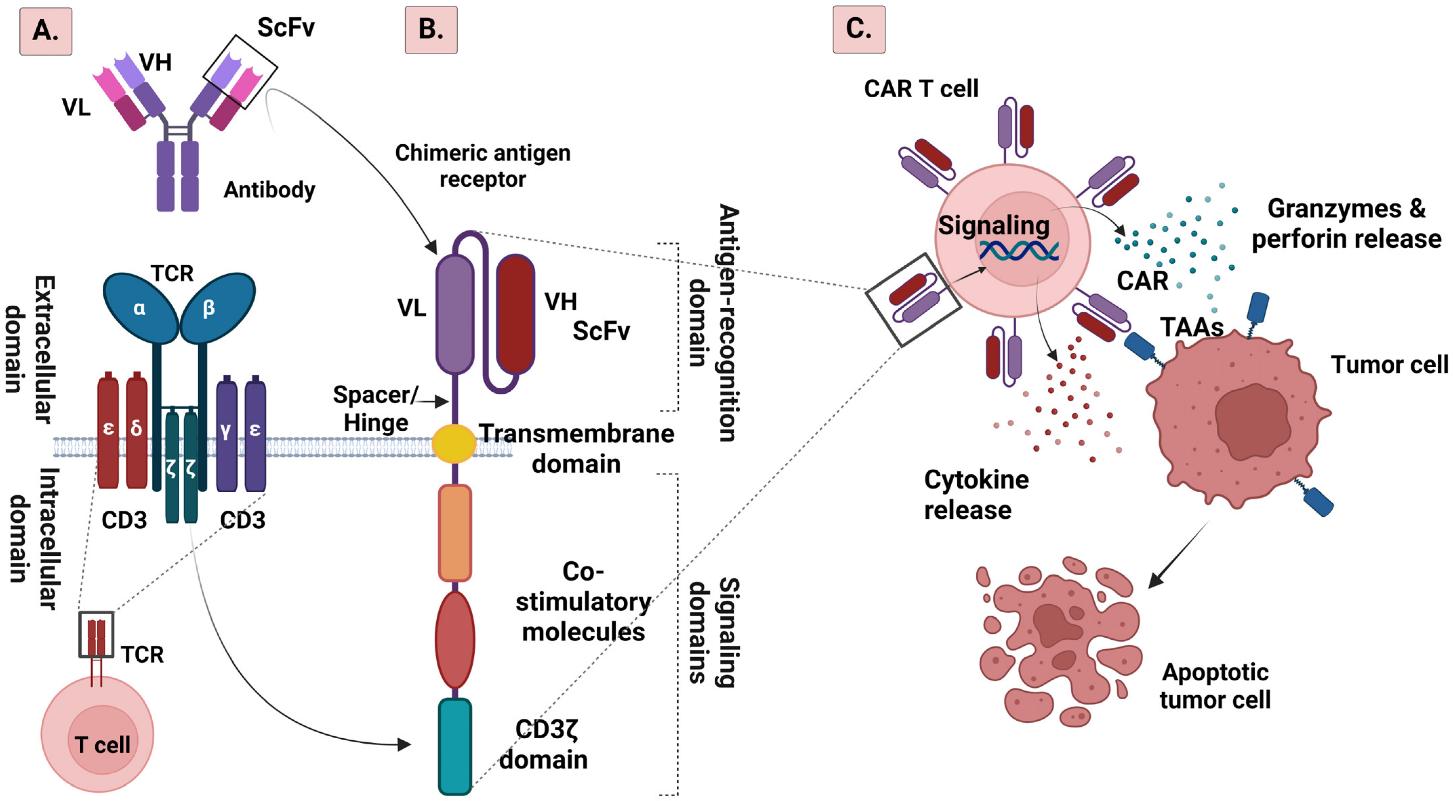 Fig.2 Schematic illustration of TCR (A) and CAR (B) structures, and mechanism of CAR T cell treatment (C) 2,4.
Fig.2 Schematic illustration of TCR (A) and CAR (B) structures, and mechanism of CAR T cell treatment (C) 2,4.
The extracellular domain typically incorporates a single-chain variable fragment (scFv) derived from a monoclonal antibody. This domain determines the receptor's targeting specificity and binding affinity. Recent innovations have explored alternative binding domains, such as single-domain antibodies, offering unique advantages in terms of size, stability, and immunogenicity.
The choice of scFv significantly influences CAR performance, with factors such as binding affinity, antigen density requirements, and potential immunogenicity all playing crucial roles. Recent studies have shown that extremely high-affinity binding may not always be optimal, as it can lead to premature T cell exhaustion or increased off-tumor toxicity. Engineers must carefully balance these parameters when designing new CARs.
The hinge or spacer region, often derived from CD8α or IgG4, provides crucial flexibility and optimal distance between the T cell and target cell. The length and composition of this region significantly influence receptor functionality, particularly when targeting membrane-proximal versus membrane-distal epitopes.
Recent structural studies have revealed that the spatial relationship between the CAR and its target antigen profoundly affects signal transduction efficiency. Optimal spacer length appears to be antigen-dependent, with membrane-proximal antigens generally benefiting from shorter spacers and membrane-distal antigens requiring longer ones. This understanding has led to the development of tailored spacer designs for specific target antigens.
The transmembrane domain, typically derived from CD8 or CD28, anchors the receptor in the cell membrane and plays a crucial role in receptor oligomerization and signal transduction. Recent structural studies have revealed that this domain's specific amino acid composition influences receptor clustering and subsequent signal strength.
The importance of the transmembrane domain extends beyond mere membrane anchoring. Its sequence can affect CAR stability, expression levels, and interaction with endogenous signaling molecules. Some designs incorporate modified transmembrane domains to enhance receptor clustering or promote interaction with specific membrane-associated proteins.
The intracellular signaling domain orchestrates T cell activation upon antigen recognition. Its composition has evolved significantly through different chimeric antigen receptor generations, reflecting our deepening understanding of T cell biology and signal transduction pathways. This domain typically includes the CD3ζ chain, which contains three immunoreceptor tyrosine-based activation motifs (ITAMs) essential for initiating T cell activation.
The inaugural chimeric antigen receptor design featured a remarkably straightforward yet revolutionary approach to T cell engineering. These receptors incorporated only the CD3ζ chain as their signaling component, mirroring the natural T cell receptor (TCR) signaling pathway. The pioneering work in this field, particularly by Carl June CAR-T research group, demonstrated the first successful redirection of T cell specificity using these chimeric receptors.
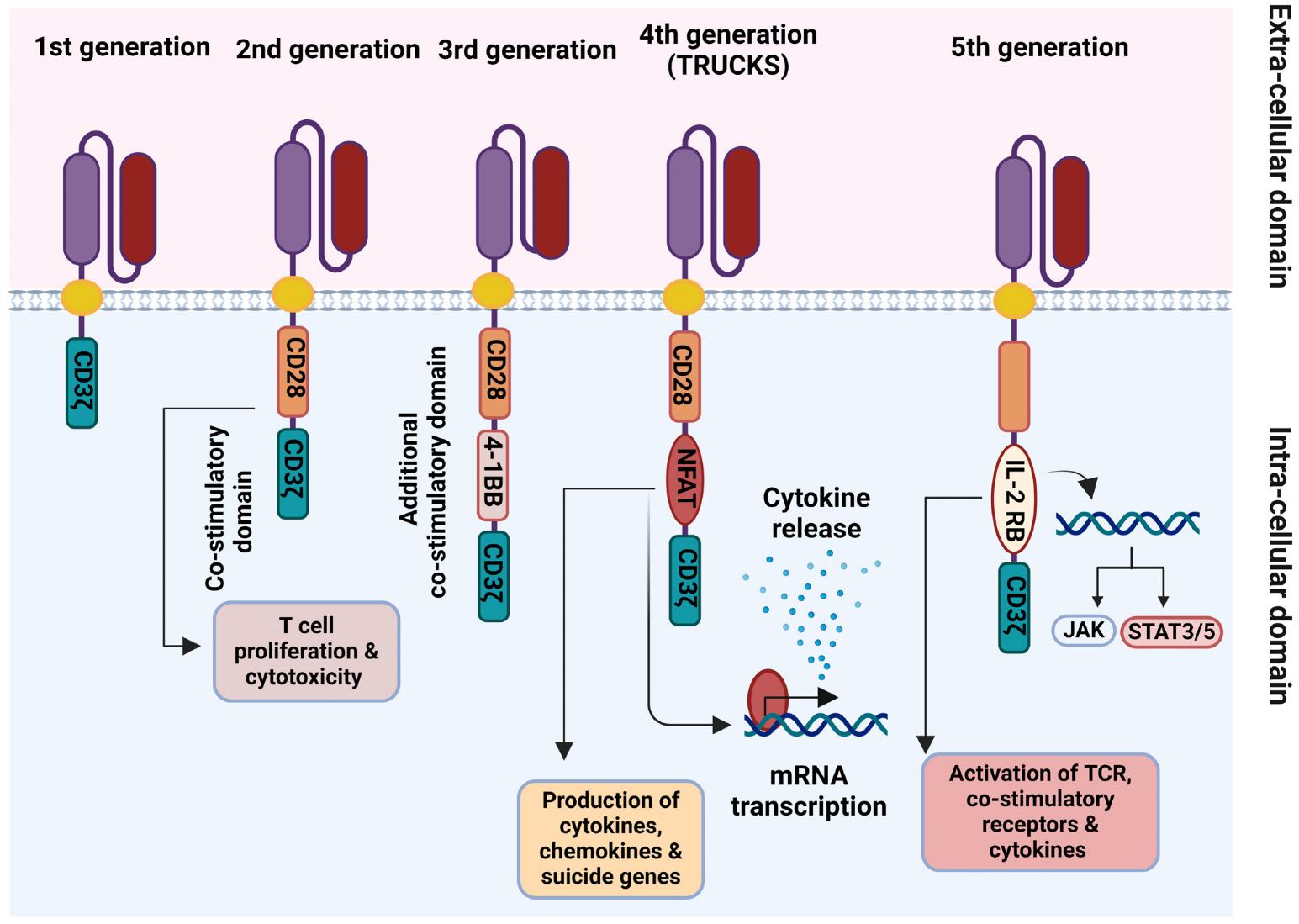 Fig.3 Schematic representation of the 1st–5th generations of chimeric antigen receptors (CARs) 2,4.
Fig.3 Schematic representation of the 1st–5th generations of chimeric antigen receptors (CARs) 2,4.
First-generation CARs showed promising results in laboratory settings, successfully demonstrating specific target cell lysis in vitro. These early experiments validated the basic concept of using engineered receptors to redirect T cell specificity and confirmed that modified T cells could recognize and eliminate target cells expressing specific antigens.
The primary challenge stemmed from insufficient T cell activation in clinical settings, leading to reduced proliferation and survival in vivo. The absence of co-stimulatory signals resulted in T cell anergy or exhaustion, limiting their therapeutic potential. Clinical trials revealed that while engineered T cells could traffic to tumor sites and exhibit some anti-tumor activity, they failed to persist long-term in patients.
Second-generation CARs marked a pivotal advancement by incorporating a co-stimulatory domain alongside CD3ζ. This modification dramatically enhanced T cell functionality across multiple parameters. The most commonly utilized co-stimulatory domains include CD28 and 4-1BB (CD137), each imparting distinct characteristics to the engineered T cells.
CD28-based constructs typically generate more rapid and intense responses, characterized by robust proliferation and cytokine production. However, these cells often exhibit shorter persistence due to accelerated exhaustion. In contrast, 4-1BB-containing CARs promote enhanced T cell survival and memory formation, leading to improved long-term persistence but potentially slower initial responses.
The success of CD19-targeted second-generation CARs in treating B-cell malignancies represents a watershed moment in cancer immunotherapy. These treatments demonstrated unprecedented response rates in patients with relapsed/refractory disease, leading to multiple FDA approvals. The clinical success sparked intense research into optimizing co-stimulatory domains and exploring new target antigens.
Third-generation CARs represent an ambitious attempt to further enhance T cell functionality by incorporating two co-stimulatory domains. These receptors typically combine CD28 with either 4-1BB or OX40, aiming to synergistically improve T cell activation, proliferation, and persistence. The underlying hypothesis suggested that multiple co-stimulatory signals would better mimic natural T cell activation.
The clinical benefits of this approach remain somewhat controversial. While some studies demonstrate enhanced anti-tumor activity, others suggest that the additional complexity might lead to tonic signaling or accelerated T cell exhaustion. The optimal combination and arrangement of co-stimulatory domains continue to be active areas of investigation.
Recent studies have explored novel combinations of co-stimulatory domains, including less common molecules such as ICOS and CD27. These investigations have revealed complex interactions between different signaling pathways and their impact on T cell function. Some researchers have also examined the potential benefits of incorporating inhibitory domains to create more finely tuned responses.
Fourth-generation CARs incorporate an additional transgene expression cassette, enabling engineered T cells to produce and secrete specific proteins, typically cytokines or other immunomodulatory molecules, upon chimeric antigen receptor activation.
This generation addresses the challenge of immunosuppressive tumor microenvironments by enabling local delivery of therapeutic proteins. Common approaches include IL-12, IL-18, or CCL19 secretion to enhance T cell function and recruit additional immune cells. Some designs incorporate inducible suicide genes or safety switches, providing enhanced control over the engineered T cells.
The fourth-generation CARs offer several advantages over conventional CAR designs. By localizing cytokine production to the tumor site, these cells can potentially overcome immunosuppressive mechanisms while minimizing systemic toxicity. The ability to recruit and activate additional immune cell types may be particularly valuable in solid tumor applications, where the establishment of a productive immune response often requires coordinated activity of multiple cell types.
Recent innovations in fourth-generation designs include sophisticated control mechanisms for transgene expression. These include systems responsive to small molecules, hypoxia, or specific metabolic conditions found in the tumor microenvironment. Some designs incorporate feedback loops to modulate cytokine production based on the local inflammatory environment.
The latest evolution in CAR technology integrates sophisticated signaling modifications to enhance T cell functionality and control. These designs often incorporate constitutively active or modified signaling domains, such as truncated cytokine receptors or synthetic signaling components. A notable example includes the incorporation of constitutively active STAT3 signaling domains, which provides enhanced proliferation and survival signals to the engineered T cells.
Fifth-generation CARs introduce revolutionary approaches to antigen recognition and response modulation. Some designs incorporate split signaling domains that require dual antigen recognition for full activation, improving specificity and reducing off-tumor effects. This advancement addresses one of the key challenges in CAR therapy - the balance between efficacy and safety. The development of logic gated CAR T cells which can recognize multiple antigens in defined combinations, represents a significant step toward more precise targeting of cancer cells.
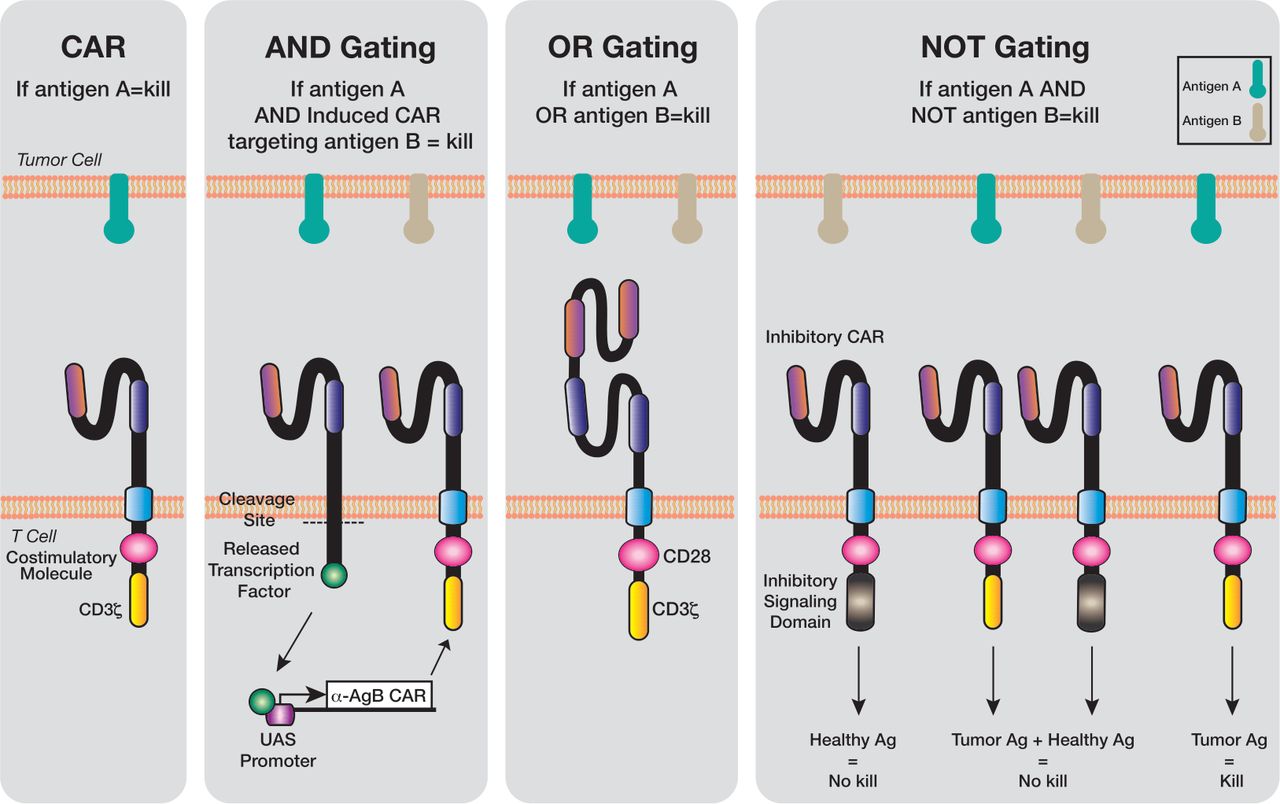 Fig.4 Logic gating technology has been applied to genetically engineered T cells3,4.
Fig.4 Logic gating technology has been applied to genetically engineered T cells3,4.
The integration of synthetic biology principles has led to increasingly sophisticated control mechanisms. These include: 1) Environmental sensor modules that detect specific conditions in the tumor microenvironment; 2) Programmable response thresholds based on antigen density; 3) Metabolic sensors that respond to tumor-specific metabolic conditions; 4) Synthetic promoter systems allowing for precise control of gene expression; 5) These innovations enable context-dependent activation and more nuanced therapeutic responses.
The development of universal CAR-T platforms represents a major focus of fifth-generation design. These "off-the-shelf" products aim to overcome the limitations of autologous CAR-T cell therapy through several innovations: 1) Novel gene editing approaches to eliminate alloreactivity; 2) Incorporation of multiple safety switch mechanisms; 3) Development of standardized manufacturing processes; 4) Integration of resistance to host immune responses. These advances could significantly reduce production costs and improve treatment accessibility.
Fifth-generation CARs incorporate advanced control systems that go beyond traditional safety switches: 1) Small molecule-dependent activation systems; 2) Light-controlled (optogenetic) CAR activation; 3) Temperature-sensitive control elements; 4) Bispecific adapter molecules for tunable targeting. These mechanisms provide unprecedented control over CAR-T cell activity and enable rapid intervention if necessary.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION