Antibody Glycosylation Analysis Service
What is Antibody Glycosylation?
The glycosylation process is a vital post-translational modification that determines antibodies' functional characteristics and stability while maximizing their therapeutic capabilities. The sugar chains determine more than half of antibody functionality which makes their precise analysis essential. Creative Biolabs develops antibody glycosylation analysis services to tackle these intricate challenges so you can create antibodies with improved performance and safety. Our customized solutions deliver actionable insights into each aspect of glycosylation whether you're conducting early-stage research or working through late-phase drug development.
Types of Antibody Glycosylation
Antibodies undergo two main types of glycosylation, each with distinct impacts on their behavior:
- N-Glycosylation: The most prevalent form, occurring at the consensus sequence Asn-X-Ser/Thr (where X ≠ Pro). In IgG, this is predominantly at Asn297 in the CH2 domain of the Fc region, a site crucial for maintaining structural integrity and mediating interactions with Fc receptors. The glycans here can be high-mannose, hybrid, or complex, each bringing unique functional effects—think sialylation for extended half-life or afucosylation for boosted ADCC activity.
- O-Glycosylation: Less common in antibodies, attaching sugars to serine or threonine hydroxyl groups. While sparse, these modifications can still influence antibody stability and immune recognition, particularly in the hinge or variable regions.
How Glycosylation Shapes Antibody Function
The sugar chains decorating antibodies act as molecular switches, controlling:
- Glycans like G0F enhance complement-dependent cytotoxicity (CDC), while afucosylation ramps up antibody-dependent cellular cytotoxicity (ADCC)—critical for cancer therapies.
- Sialylated glycans shield antibodies from clearance, prolonging their circulation, whereas high-mannose structures can accelerate degradation.
- Non-human glycans, such as α-1,3-galactose, can trigger unwanted immune responses, making glycan profiling essential for minimizing safety risks.
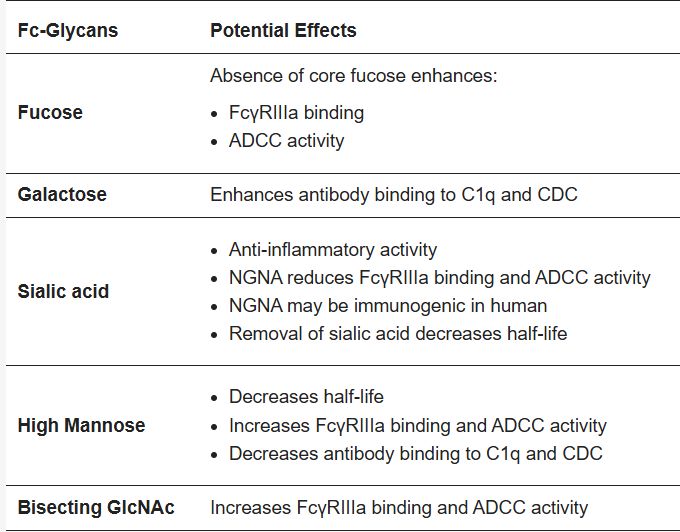 Fig.1 The most prevalent Fc-glycans have several potential effects on mAbs.1
Fig.1 The most prevalent Fc-glycans have several potential effects on mAbs.1
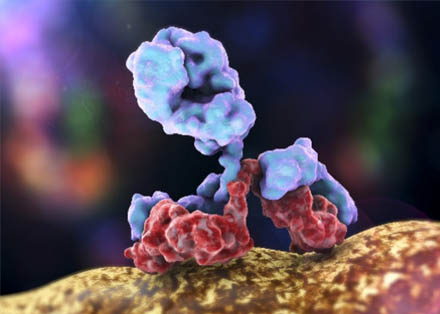
Key Glycosylation Sites in IgG
The Fc region's Asn297 is the primary glycosylation hotspots in IgG, with over 90% of therapeutic antibodies carrying glycans here. This site is tightly linked to Fc folding and effector interactions—even minor changes in glycan structure (e.g., fucose loss or galactose addition) can drastically alter binding to FcγRs or C1q. While less common, secondary sites in the Fab region (e.g., Asn in the CDR loops) can affect antigen binding and specificity, though their analysis requires specialized techniques to avoid masking by primary glycans.
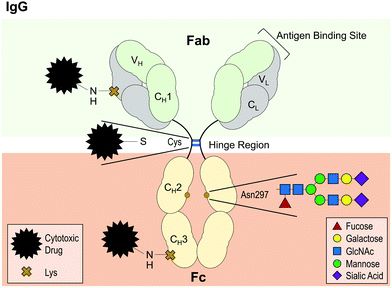 Fig.2 IgG antibody structure with highly conserved glycosylation site at asparagine (Asp) 297.2
Fig.2 IgG antibody structure with highly conserved glycosylation site at asparagine (Asp) 297.2
Our Holistic Analysis Workflow
At Creative Biolabs, we've streamlined the analysis process to deliver clarity without compromise. Our workflow combines cutting-edge techniques with rigorous quality control:
1. Sample Preparation
Whether you provide cell culture supernatants, serum, or tissue lysates, we optimize extraction to preserve glycan integrity. For tissue samples, we use gentle homogenization and ultracentrifugation to isolate antibodies without denaturation—a must for accurate downstream analysis.
2. Glycan Release
We employ PNGase F for N-glycan release, a highly specific enzyme that cleaves the glycosidic bond at Asn, converting it to Asp without damaging the peptide backbone. For tricky structures like α1-3-fucose-core glycans, we pair it with PNGase A to ensure complete release.
3. Labeling & Derivatization
Free glycans are notoriously challenging to analyze due to low ionization efficiency. That's why we use proven labeling strategies:
- Reducing-End Modification: Fluorescent tags (e.g., APTS) for HPLC/CE detection, or isotopic labels (e.g., SILAC, TMT) for quantitative MS analysis.
- Permethylation: Neutralizes acidic groups, improving MALDI-MS sensitivity and enabling separation of complex glycan isomers.
4. Separation & Detection
- Mass Spectrometry (MS): Our gold-standard method for structural elucidation, offering high-resolution analysis of glycan composition (e.g., sialic acid count, fucose presence) and site-specific occupancy. We use both ESI-MS for dynamic range and MALDI-MS for high-throughput screening.
- HILIC-LC: Separates glycans by hydrophilicity, ideal for quantifying glycoforms like G0, G1, G2 in IgG Fc.
- Capillary Electrophoresis (CE): Resolves challenging isomers (e.g., sialic acid linkages) with picomolar sensitivity, perfect for low-volume samples.
5. Data Interpretation
Our bioinformatics team doesn't just report numbers—we contextualize them. Using tools like PCA and PLS, we highlight glycan variations linked to functional changes, such as how a shift in galactosylation impacts CDC activity. For therapeutic development, we even perform ROC analysis to assess glycan biomarkers for clinical utility.
Know More about Anti-Glycan Antibody Detection Services
Advanced Techniques for Complex Challenges
Site-Specific Glycosylation Analysis
Going beyond bulk glycan profiling, our site-specific approach identifies which Asn residues are glycosylated and how their occupancy varies between samples. Using enrichment strategies like lectin affinity chromatography (high specificity for N-glycopeptides) and LC-ESI-MS/MS, we map glycosylation sites with up to 10,000-site detection capacity—critical for characterizing heterogeneous antibody populations.
Glycoengineering Support
Pair our analysis with glycoengineering services to iterate faster:
- Cell Line Optimization: Identify glycosyltransferases to knockout/overexpress for desired glycan profiles (e.g., afucosylated cells for enhanced ADCC).
- Enzymatic Modification: In vitro glycan trimming/adding (e.g., sialyltransferases for extending half-life) with MS-guided quality control.
- Metabolic Labeling: Incorporate unnatural sugars to track glycan biosynthesis, a powerful tool for mechanistic studies.
Why Choose Creative Biolabs?
-
Unmatched Expertise in Glycosylation Biology
Our team combines decades of experience in glycoprotein science with a deep understanding of antibody engineering. We don't just follow protocols—we innovate them, troubleshooting tricky samples (e.g., low-abundance therapeutic antibodies in serum) with creative solutions.
-
State-of-the-Art Platforms
From Orbitrap MS for ultra-high resolution to HILIC columns optimized for glycan retention, our tools are chosen for precision. We validate every method rigorously, ensuring CVs <10% for quantitative assays and >95% site identification accuracy.
-
High-Throughput & Custom Solutions
Whether you need batch-to-batch QC for manufacturing or a bespoke assay for novel antibody formats (bispecifics, ADCs), we scale our services to your needs. Our labs handle everything from serum samples to tumor tissues, with turnaround times tailored to your project's urgency.
-
Partner-Centric Approach
We know your project is unique. That's why we offer fully customizable workflows—whether you need raw data delivery, full statistical analysis, or a consultative report linking glycan profiles to mechanistic insights, we adapt to your goals.
Applications
- Early Discovery: Characterize lead candidates for glycan homogeneity, ensuring stable developability.
- Preclinical Optimization: Use glycoengineering to tune effector functions (e.g., afucosylation for cancer drugs, sialylation for anti-inflammatory antibodies).
- Process Development: Monitor cell line stability, upstream/downstream changes, and formulation effects on glycosylation.
- Regulatory Submissions: Provide robust data packages for CMC sections, including site occupancy, glycoform distribution, and lot-to-lot consistency.
At Creative Biolabs, our antibody glycosylation analysis services bridge the gap between complex glycan structures and tangible therapeutic benefits. Whether you're optimizing an existing candidate or developing the next-generation antibody, our team is here to ensure every glycosylation detail works in your favor. From N-glycan release to glycoengineering support, we offer end-to-end solutions that accelerate your journey from bench to bedside. Contact us today to discuss how our expertise can elevate your antibody project. Let's turn glycan complexity into your competitive edge.
References:
- Boune, Souad, et al. "Principles of N-linked glycosylation variations of IgG-based therapeutics: pharmacokinetic and functional considerations." Antibodies 9.2 (2020): 22. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/antib9020022
- Lundahl, Mimmi LE, et al. "Aggregation of protein therapeutics enhances their immunogenicity: causes and mitigation strategies." RSC chemical biology 2.4 (2021): 1004-1020. Distributed under Open Access license CC BY 3.0, without modification. https://doi.org/10.1039/D1CB00067E