Custom Anti-sLeX Antibody Development for Cancer Metastasis Research
Developing effective antibodies against complex glycans is a significant challenge in modern biology. These sugar molecules, known as glycans, play crucial roles in cancer progression. Metastasis, the spread of cancer cells from a primary tumor to distant organs, is the leading cause of mortality in cancer patients. Understanding how cancer cells travel and form new tumors is one of the most critical goals in modern oncology. At the center of this complex process is a specific molecule: sialyl Lewis X. This small glycan is overexpressed on the surface of many types of cancer cells. It acts as a critical adhesion molecule, allowing circulating tumor cells to bind to the walls of blood vessels. This binding is the first and most essential step in the metastatic cascade. To study this mechanism, block this interaction, or identify these dangerous cells, researchers need a highly specific and reliable tool: a sialyl Lewis X antibody. However, developing antibodies against glycans, such as sialyl-Lewis X, is extremely challenging. These targets are known to be difficult. They are poor at triggering an immune response, and they often look very similar to other glycans, leading to cross-reactivity issues. Creative Biolabs specializes in developing high-affinity, high-specificity custom anti-TACA antibodies against the most challenging targets. Our custom anti-sLeX antibody development service provides you with a precision-engineered tool, fully validated for your specific metastasis research application.
Sialyl Lewis X as a Critical Mediator for Metastasis
To appreciate its role in cancer, we first need to understand its vital job in the healthy body. Sialyl Lewis X (sLeX), also known as CD15s, is a specific tetrasaccharide composed of a sialic acid, fucose, and an N-acetyllactosamine. It often displays on the cell surface, attached to O-glycans. It is essential for cell-to-cell recognition processes. Its most critical function is in the immune system. sLeX is the key structure that binds to selectin proteins (E-selectin, P-selectin, and L-selectin) on blood vessel walls. This binding enables immune cells, such as granulocytes and monocytes, to slow down, roll, and then exit the bloodstream to reach sites of inflammation. This sLeX-mediated process is essential for a normal inflammatory response.
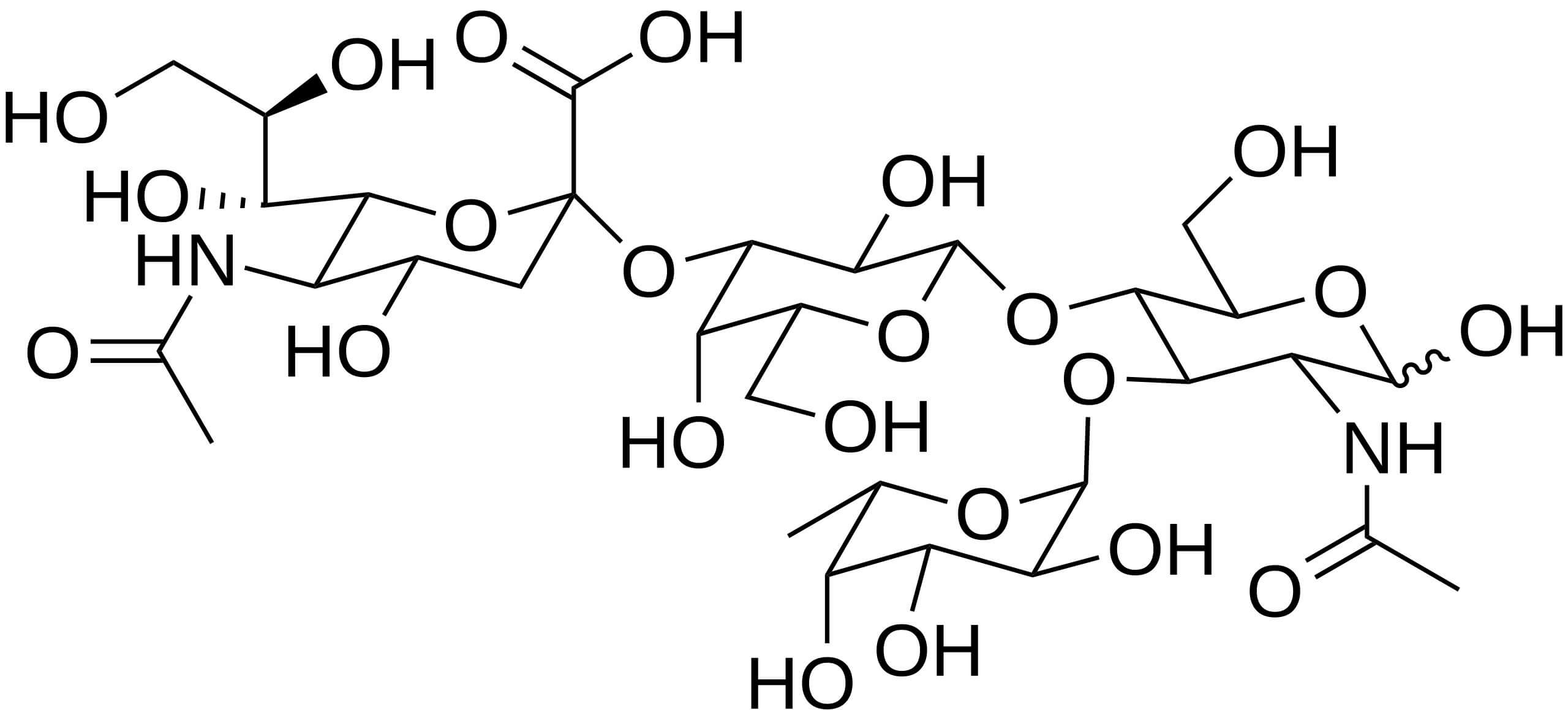 Fig.1 The structure of sialyl Lewis x (sLeX).1
Fig.1 The structure of sialyl Lewis x (sLeX).1
How Cancer Exploits the sLeX Pathway
Many aggressive cancers, including those of the colon, breast, lung, stomach, and pancreas, have learned to utilize this process. They exploit this adhesion mechanism for their own purposes.
- Over-Expression: These tumor cells begin to produce and display massive amounts of sialyl-Lewis X on their surface.
- Adhesion: When a circulating tumor cell enters the bloodstream, its sLeX glycans bind tightly to the E-selectin and P-selectin proteins expressed on endothelial cells, often at distant sites.
- Extravasation: This binding anchors the tumor cell to the blood vessel wall. This adhesion is the essential first step that allows the cell to penetrate the vessel wall and invade a new tissue.
- Metastasis: Once inside the new tissue (like the liver or lungs), the cancer cell can proliferate and form a new, deadly secondary tumor.
Clinical data strongly support this. High expression of sialyl-Lewis X in patient tumors is significantly correlated with increased lymph node metastasis, liver metastasis, and overall poor prognosis. This makes the sLeX-selectin interaction a high-value target for metastasis research. A sialyl-Lewis X antibody that can specifically bind to sLeX or block this interaction is one of the most powerful research tools you can have.
The Challenge: Why Are Glycan Antibodies Difficult to Make?
If sLeX is such a clear target, why is developing these antibodies not routine? The answer lies in the unique challenges of glycobiology. Standard antibody development protocols fail when faced with these challenges. Success requires a specialized, custom approach that starts with expert antigen design and ends with rigorous, functional validation.
Low Immunogenicity
Sugars are small, simple molecules compared to large proteins. The immune systems of animals (like mice or rabbits) often do not recognize them as "foreign" and fail to produce a strong antibody response.
High Cross-Reactivity
The sLeX structure is very similar to other common glycans, such as sialyl-Lewis A (sLeA), Lewis X (LeX), and Lewis Y (LeY). An antibody that accidentally binds to these other structures will produce confusing, inaccurate, and unusable data. Specificity is paramount.
Structural Heterogeneity
In a tumor, sLeX does not exist in isolation. It is attached to a protein or a lipid. An antibody must be able to recognize the sLeX glycan in this natural, complex presentation, not just as a synthetic sugar in a test tube.
Need for Functional Validation
It is not enough for an antibody to bind to sLeX. For metastasis research, you need to know if it can function. Does it block the binding to E-selectin? Does it stop cells from adhering?
Our Solution: A Five-Step Custom Development Workflow
We have developed a comprehensive, end-to-end service designed to address the specific challenges of creating a custom sialyl-Lewis X antibody. Our process is transparent, collaborative, and tailored to your exact research goals.
This is the most crucial step. We begin with a deep consultation with your research team. What is your final application?
- Do you need an antibody for detection in IHC or flow cytometry?
- Do you need a functional blocking antibody for in vivo metastasis models?
- Are you exploring sLeX as a target for an ADC?
Based on your goals, our glycobiology experts will design the perfect immunogen to generate the antibodies you need. Our options include:
- Synthetic Glycan Conjugates: We use high-purity, chemically synthesized sLeX tetrasaccharides. To overcome low immunogenicity, we covalently link these glycans to powerful carrier proteins. This method enhances the immune response against the glycan.
- Glycopeptide/Glycolipid Antigens: To mimic the natural presentation of sLeX, we can synthesize the glycan on a peptide or lipid backbone. This often results in antibodies that have higher affinity for sLeX on the cell surface.
- Cell-Based Immunogens: In some cases, the best antigen is the one from the tumor itself. We can use membrane extracts or glycolipid preparations from sLeX-positive cancer cell lines to immunize animals. This approach was used successfully to generate antibodies against the related glycan.
We employ multiple generation technologies, allowing us to select the best path for your project.
- Advanced Hybridoma Development: This classic method is reliable for generating high-affinity murine monoclonal antibodies. We use specialized immunization protocols and adjuvants to maximize the immune response against the sLeX glycan.
- Phage Display: This is an incredibly powerful in vitro alternative. We can screen our massive, pre-built human and synthetic antibody libraries to find binders for sLeX. This technology is high-speed and enables highly controlled selection.
This phase is dedicated to identifying promising antibody candidates from the generation phase. For hybridomas, we use high-throughput ELISA screening to test thousands of hybridoma clones for binding against the sLeX antigen. Positive clones are selected, subcloned, and expanded for further analysis. For phage display, which truly excels at screening, we employ sophisticated selection protocols. This includes negative selection by panning the library against related glycans (like sLeA) first. This step removes cross-reactive binders. We then perform positive selection against sLeX to isolate highly specific antibodies.
An antibody is only as good as its validation data. We provide a comprehensive data package to demonstrate that your antibody is effective for its intended application.
- We confirm that the antibody clones bind strongly to the sLeX antigen. We use SPR to provide quantitative data on binding affinity.
- We test your antibody against a custom glycan array. This array includes sLeX and all its close structural relatives (sLeA, LeX, LeY, etc.). We provide you with precise data showing your antibody binds only to sialyl-Lewis X.
- We test the antibody's ability to bind to real cancer cells. We use sLeX-positive cell lines and sLeX-negative control cells. This confirms the antibody recognizes the native glycan on the cell membrane.
- We perform functional assays to demonstrate the effectiveness of the antibody.
Once we have a validated, high-performance clone, we tailor it to suit your specific research needs.
- Chimerization & Humanization: If you plan to use the antibody in mouse models (xenografts), a murine antibody can have a long half-life. But for other in vivo studies or pre-therapeutic development, we can convert your mouse antibody into a chimeric (mouse-human) or fully humanized antibody.
- Isotype Switching: Do you need a specific effector function? We can switch the antibody's isotype to human IgG1 (for high effector function) or IgG2/IgG4 (for less effector function) to match your experimental design.
- Large-Scale Production: We provide high-purity, low-endotoxin antibody production at any scale, from milligrams for in vitro testing to multi-gram batches for extensive in vivo metastasis research.
Ready-to-Use sialyl-Lewis X antibody Products
For researchers who need a validated tool immediately, a custom development project may not be the fastest path. We also offer an extensive catalog of ready-to-use sialyl-Lewis X antibody products. Our portfolio includes a diverse selection of high-quality monoclonal antibodies. Many are already validated for standard applications, such as flow cytometry, immunohistochemistry (IHC), and ELISA. These antibodies are in stock and ready to ship, allowing you to start your critical metastasis research without delay.
-
Mouse Anti-Sialyl Lewis X Monoclonal Antibody (CGYJ083)(CAT#: AGM-209YJ)Online InquiryHost: MouseAntibody Isotype: IgM, κSpecies Reactivity: HumanApplication: IHC, FC, ELISA, WB, Inhib
-
Host: MouseAntibody Isotype: IgMSpecies Reactivity: HumanApplication: FC, IHC, RIA
Why Partner with Us?
- Our team has specific expertise in overcoming the challenges of glycan immunogenicity and specificity.
- We deliver tools that are proven to work well in the assays.
- We are dedicated to helping you choose the most suitable methods for your antibody development according to your requirements.
- We are collaborative. Your project manager and scientific team will be in close contact with you from antigen design to final antibody delivery, ensuring the tool we build perfectly matches your needs.
Related Services
The link between sialyl-Lewis X and cancer metastasis is one of the most promising frontiers in oncology research. But to explore it, you need a tool that is precise, powerful, and reliable. A generic, off-the-shelf antibody will not be enough. You need a custom sialyl-Lewis X antibody built for your specific question. Contact our scientists today, and let's discuss your metastasis research goals and begin designing the exact antibody you need to make the next breakthrough.
Published Data: Anti-sLeX Antibody in Action
While our service focuses on cancer, the mechanism of sLeX is fundamental to other diseases. A key study on allergic asthma highlights the therapeutic potential of an anti-sLeX antibody. In this study, researchers employed a murine model of asthma, where leukocyte infiltration into the lungs leads to inflammation. This infiltration is mediated by sLeX on leukocytes binding to P-selectin and E-selectin on endothelial cells. The study introduced a specific anti-sLeX monoclonal antibody, mAb F2. The results were definitive. Administration of the F2 antibody significantly suppressed eosinophil infiltration into the lungs. It also reduced downstream allergic responses, including the production of Th2 cytokines and IgE levels. This demonstrates the potent ability of a sialyl-Lewis X antibody to functionally block the sLeX-selectin interaction in vivo, preventing pathological cell migration. This same principle of blocking cell adhesion is the central goal for anti-metastasis therapeutic research.
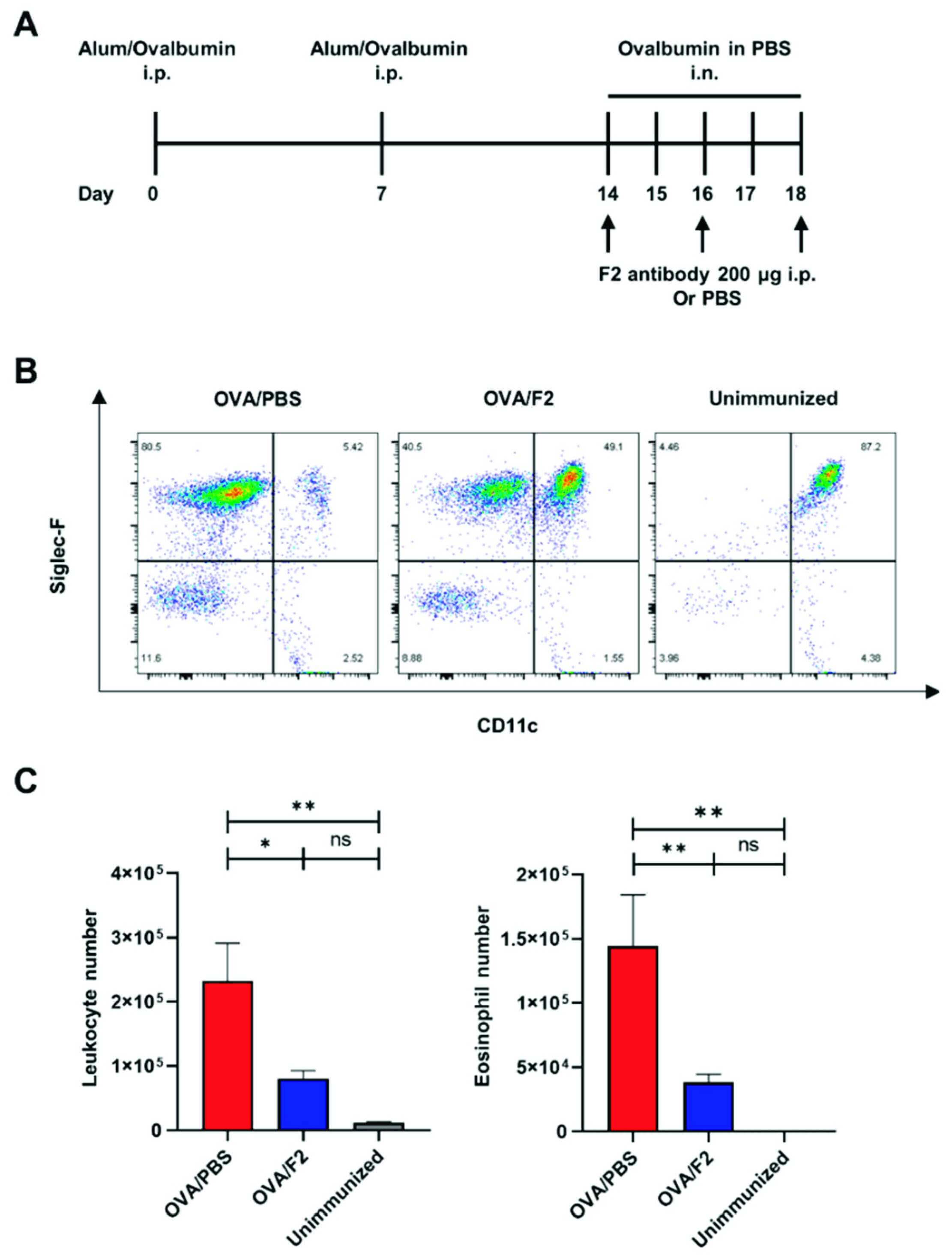 Fig.2 Anti-sLeX mAb F2 suppression of leukocyte infiltration in BALF.2
Fig.2 Anti-sLeX mAb F2 suppression of leukocyte infiltration in BALF.2
References:
- Distributed under Public Domain, from Wiki, without modification.
- Xiong, Wei, et al. "Therapeutic effects of an anti-sialyl Lewis X antibody in a murine model of allergic asthma." International Journal of Molecular Sciences 22.18 (2021): 9961. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/ijms22189961
Supports
- TACAs Overview
- Guide to Blood Group Antigens
- Comparing sLeA and sLeX Roles in Cancer
- CA19-9 as a Pancreatic Cancer Biomarker
- Lewis Antigen System Overview
- TACA-Targeted ADCs, CAR-Ts, and RICs
