Globoside (Gb4) as a Novel Antiviral Target: A New Model for B19V Endosomal Escape
For decades, scientists have been working to map the complex relationship between viruses and the host cells they infect. A central part of this work is understanding the "key" a virus uses to unlock a cell. For Human Parvovirus B19 (B19V), the pathogen responsible for the common childhood illness known as "fifth disease" and severe complications in high-risk patients, the key was long thought to be a molecule called Globoside. Globoside, also known as Gb4 or the P antigen, is a type of glycosphingolipid. This is a molecule made of complex sugars and fats found on the surface of our cells. For years, the established model was that B19V attached to Globoside on the cell surface and used it as a "front door" to get inside.
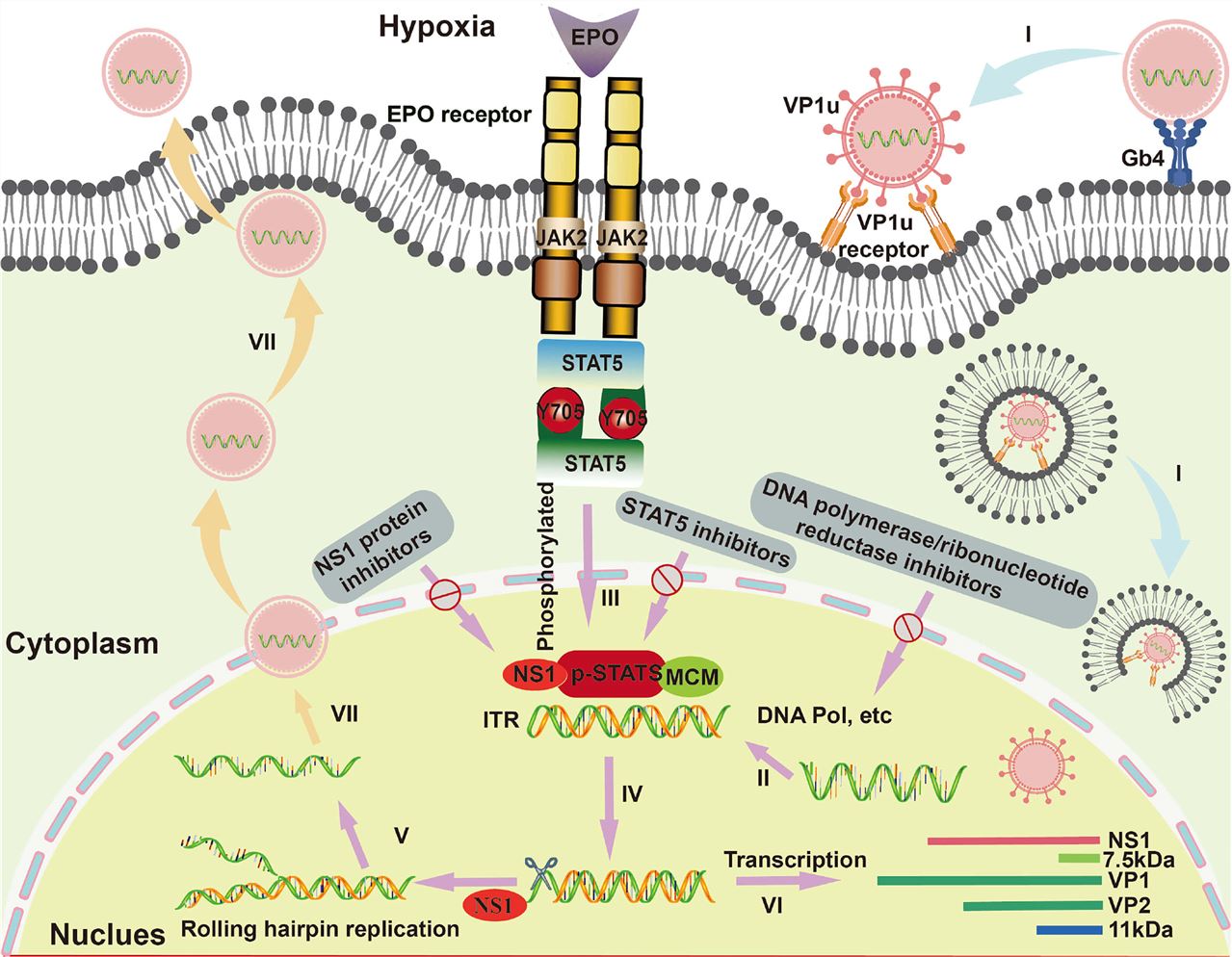 Fig.1 B19V Infection Cycle in EPCs.1,3
Fig.1 B19V Infection Cycle in EPCs.1,3
This model, however, had a major unanswered question. This question has been a significant puzzle in virology for years. B19V infection is highly specific. It almost exclusively infects and replicates in erythroid progenitor cells (EPCs) in our bone marrow. These are the cells that grow to become red blood cells. But Globoside, the supposed "front door," is not specific. It is found on the surface of many different cell types in the human body. This created a paradox: If the key (B19V) and the lock (Globoside) are present on many different "doors" (cell types), why does the virus only successfully enter and infect one specific "room" (the EPCs)? This long-standing puzzle suggested our understanding of Globoside's role was incomplete. It hinted that this molecule might be doing something far more complex than just sitting on the surface. To solve puzzles like this, researchers need extremely precise tools. They need tools that can distinguish between a molecule's function on the outside of a cell versus its function on the inside. This is a common challenge in advanced cell biology. At Creative Biolabs, our Custom Anti-Globoside Antibody Service for Cellular Receptor Studies is built to address these exact problems. We specialize in creating custom tools to help researchers find clear answers to complex biological questions.
Beyond the Surface: Globoside's Essential Intracellular Role in B19V Infection
A groundbreaking study has finally provided a clear answer. This new research fundamentally changes our understanding of B19V infection and gives Globoside a new, critical role. Here is what the study found.
Clarifying Viral Entry: VP1uR as the Primary Receptor
First, the research confirms that B19V uses a highly specific receptor to get into its target EPCs. This receptor, known as VP1uR, is the true "front door." This discovery solves the specificity puzzle. The reason B19V only infects EPCs is that only EPCs have this specific VP1uR receptor on their surface.
A pH-Dependent Mechanism for Intracellular Receptor Switching
So, what does Globoside do? Its critical job happens after the virus is already inside the cell. When B19V enters the cell, it is wrapped in a membrane bubble called an endosome. This endosome then begins to "mature," and its internal environment becomes acidic (the pH drops). This drop in pH is the critical trigger. The study shows that this acid acts like a switch: On the surface (Neutral pH 7.2), the virus strongly binds to its entry receptor, VP1uR. Inside the endosome (Acidic pH < 6.5): The virus completely let go of the VP1uR receptor. At the same time, the acidic environment causes the virus to strongly bind to Globoside, which is also present on the inner surface of the endosome membrane. The virus literally switches its binding partner. It lets go of the "front door" handle and grabs onto a new "inner" handle (Globoside) to continue its journey. This pH-driven switch is a remarkable finding. You can see this data clearly in Fig.2C.
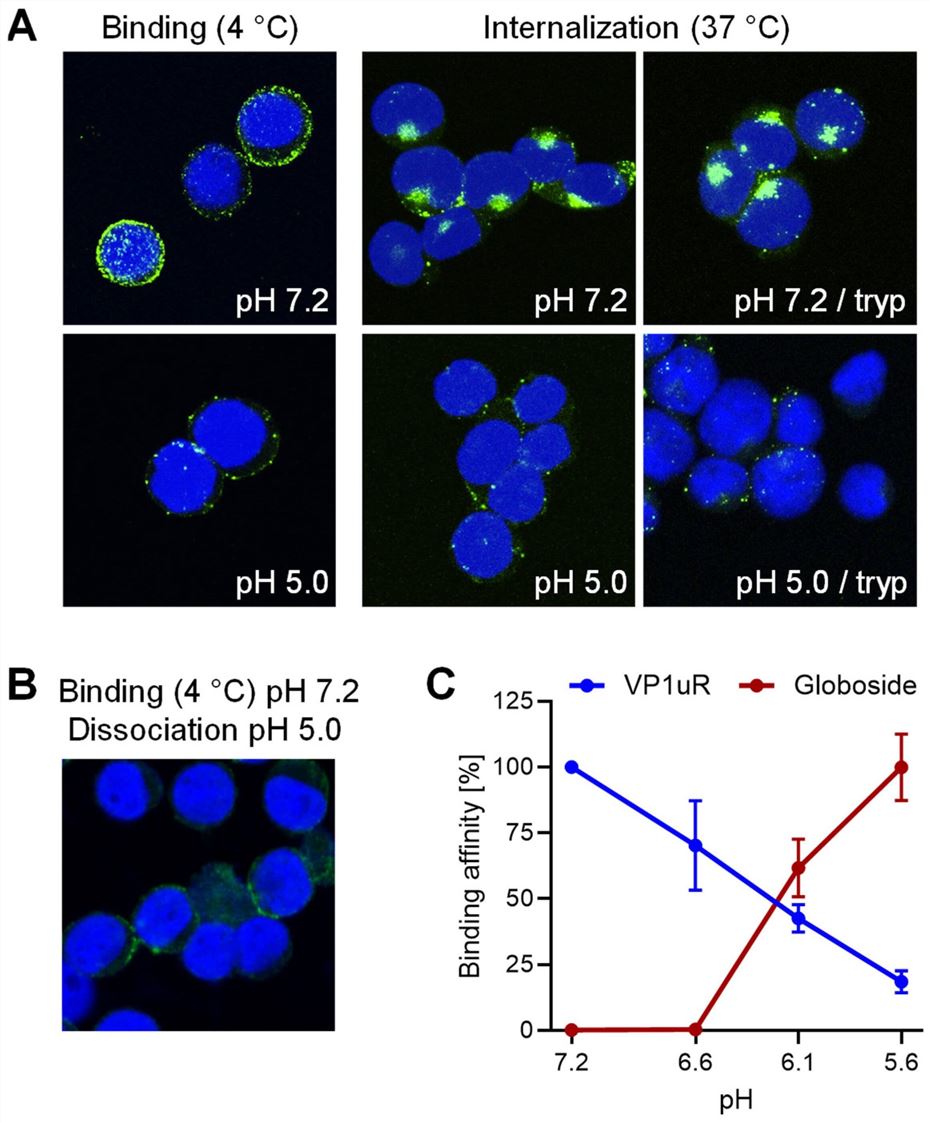 Fig.2 pH-dependent binding of b19v to globoside and VP1uR.2,3
Fig.2 pH-dependent binding of b19v to globoside and VP1uR.2,3
Evidence: Endosomal Arrest in KO Cells and PEI-Mediated Rescue
This discovery is powerful, but the researchers provided even stronger proof. They asked, 'What happens if Globoside is not there?' They used special cells that were engineered to have no Globoside (known as "Knockout" or KO cells). Here is what happened:
- Virus Entry: The virus successfully utilized the VP1uR receptor to enter the KO cells.
- Infection Failed: Once inside, the virus was completely trapped in the endosome. With no Globoside to bind to, it had no way to get out. The infection failed.
This proves that Globoside is essential for the virus to escape the endosome. The final experiment was the most definitive. The researchers took these same KO cells, where the virus was trapped, and added a chemical called PEI. PEI is a substance that can artificially break open endosomes. The infection was rescued.
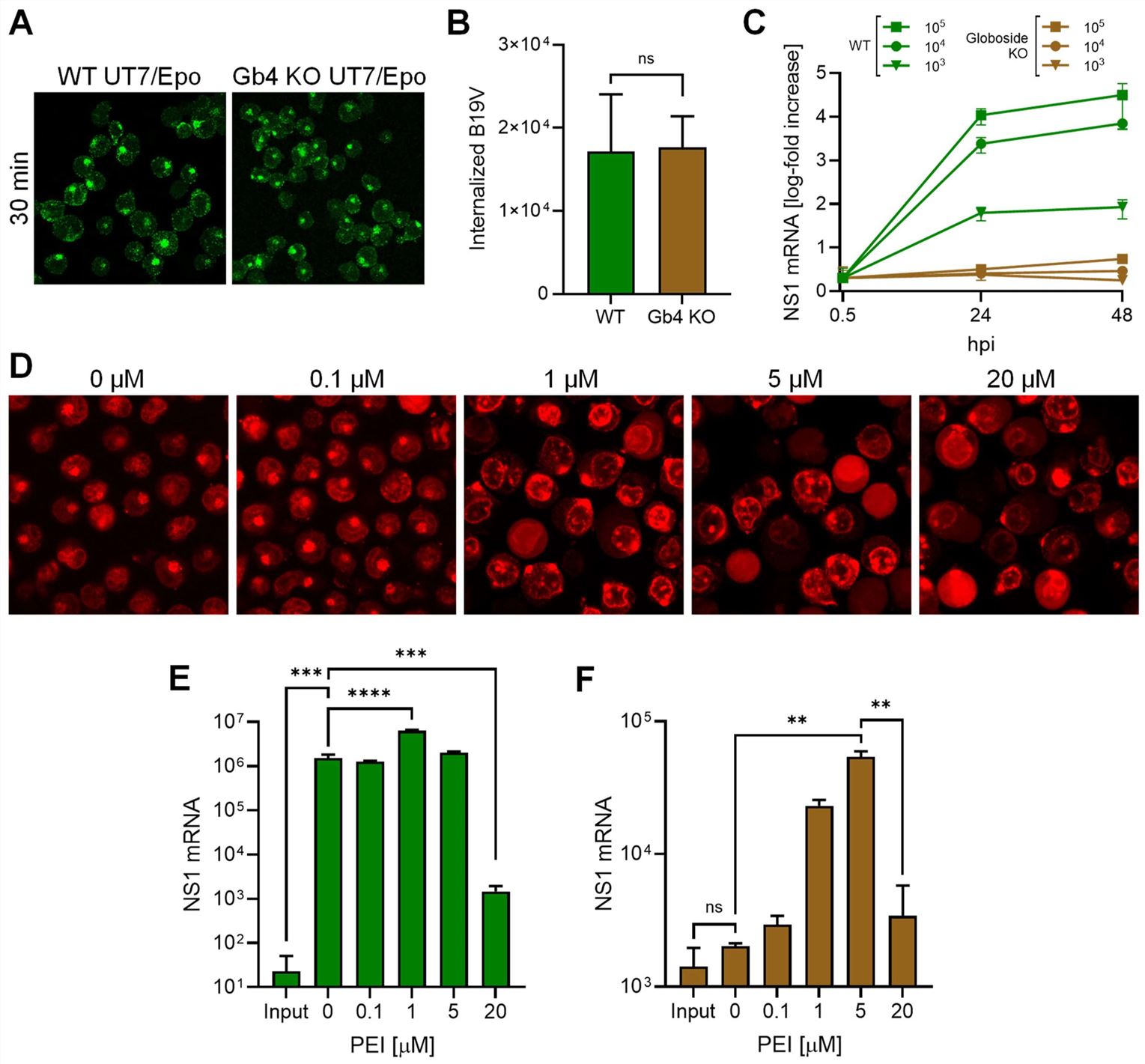 Fig.3 PEI rescues B19V infection in globoside (Gb4) KO cells.2,3
Fig.3 PEI rescues B19V infection in globoside (Gb4) KO cells.2,3
The Gb4 Pathway: A New Target for Antiviral Intervention
This discovery is incredibly exciting because it completely changes our strategy for fighting B19V. For many years, scientists have focused on developing antiviral drugs that "block the front door." The goal was to find a drug that stops the virus from attaching to the outside of the cell. This new research opens up a new and perhaps better therapeutic target. Instead of just blocking the front door, we can now design drugs to "block the escape." Imagine a drug that can enter the cell and specifically prevent the virus from binding to Globoside within the endosome. This would trap the virus in a "dead-end" pathway. The cell's natural defenses would clear the virus without ever allowing it to cause an infection. This is a new antiviral frontier. It shifts the battlefield from the cell surface to the intracellular compartments. The study also suggests that Globoside binding helps the virus travel from the endosome to another organelle, the Golgi apparatus, to complete its escape. This entire intracellular journey is now a target for research and drug discovery.
To explore this exciting new therapeutic window, researchers must first be able to study this pathway. They need to map the journey. They need to confirm this interaction in different systems. And they need tools that can work in this specific, complex intracellular environment. This requires highly specific antibodies. Our Custom Anti-Globoside (Gb3/Gb4) Antibody Service is designed for exactly this purpose. We focus on developing validated tools for advanced cellular receptor studies, enabling researchers to investigate newly discovered pathways.
Research Challenges in Mapping the Intracellular Globoside Pathway
This new research is a breakthrough, but it also highlights a significant technical challenge. Studying molecules inside a cell is significantly more challenging than learning them on the surface. This new B19V-Globoside pathway presents two specific problems for researchers.
The "Difficult Target" Problem
Glycolipids, like Globoside (Gb4) and its relative Gb3, are notoriously "difficult targets" for antibody development. They are a mix of fat (lipid) and sugar (glycan). Their structures are complex. They are embedded in membranes. These properties make them very difficult for an immune system to "see" and create a potent, specific antibody against. Many standard antibody projects against glycolipids fail.
The "Difficult Environment" Problem
This new research adds another layer of difficulty. Researchers do not just need an antibody that binds Globoside. They need an antibody that can work in the endosome, a chemically unique environment often characterized by a low pH. The antibody must be stable and bind its target here. Work for complex applications: To study this pathway, researchers need more than a simple binding test. They need antibodies for Immunofluorescence (IF). This technique allows for the visualization of the virus and the Globoside molecule traveling together within the cell. They need antibodies for Co-Immunoprecipitation (Co-IP). This technique involves pulling the virus-globoside complex out of the cell to identify other host proteins that are part of the escape plan.
These are advanced, demanding applications. Most "off-the-shelf" antibodies are not designed or validated for this kind of complex intracellular work. This is a significant technical hurdle. Using a generic antibody for this cutting-edge research can lead to months of confusing data and failed experiments. This is why our anti-glycan antibody development platform at Creative Biolabs is so valuable. We have built our expertise around solving these exact problems. We develop validated anti-globoside tools specifically for complex targets in complex cellular studies.
Custom Solution for Advanced Cellular Receptor Studies
The discovery of Globoside's intracellular role requires a new class of research tools. Creative Biolabs provides a dedicated solution: our Custom Anti-Globoside Antibody Service. This service is not a one-size-fits-all product. It is a collaborative, custom-built solution designed to solve the specific challenges highlighted by this new research.
How We Address Your Research Needs
We understand that your goal is to study a complex pathway. You need tools that are validated for your application.
We Overcome the "Difficult Target" Problem
Creative Biolabs has over 20 years of experience. We are experts in developing antibodies against "difficult" non-protein targets. We use proprietary immunization strategies and advanced antigen presentation techniques to successfully generate high-affinity antibodies against complex glycans and glycolipids, including Gb3 and Gb4.
We Deliver Tools for "Cellular Receptor Studies"
Our service name is our promise. We understand the concept of the new "intracellular receptor". We work with you to develop and validate antibodies for the specific, complex applications you need.
Our Service Can Deliver
- High-Resolution IF/ICC Antibodies: We can generate antibodies that are highly validated for immunofluorescence (IF) and immunocytochemistry (ICC). These tools will allow you to create stunning, publishable images that visually track the B19V-Globoside complex as it moves from the endosome to the Golgi.
- Antibodies for Mechanistic Studies (Co-IP): We can develop antibodies that are proven to work in Co-IP. This is essential for the next step: discovering the other host proteins that Globoside recruits to help the virus escape.
- Functional Blocking Antibodies: We can screen for functional antibodies that may be able to block the B19V-Globoside interaction physically. These are invaluable tools for in vitro assays and for validating this new pathway as a drug target.
- A Complete Panel: We offer a comprehensive range of high-titer polyclonal and high-specificity monoclonal antibodies, validated for all your standard assays, including Western Blot, ELISA, and flow cytometry.
The discovery that Globoside (Gb4) acts as an essential intracellular receptor for B19V is a breakthrough in virology and cell biology. It solves a long-standing puzzle about the virus's specificity. More importantly, it reveals a brand-new, druggable target for antiviral therapies. To explore this exciting frontier, researchers need specialized tools. High-quality, application-specific antibodies are not just helpful—they are essential for success. Creative Biolabs is your dedicated partner in developing these custom reagents. We are committed to providing the scientific community with the tools needed to study complex cellular receptors and drive the next wave of discovery. Contact our experts today to discuss your research goals. We will build the specific, validated anti-Globoside antibody tools you need to lead this new field of research.
Related Services
- Anti-Glycolipid Antibody Development
- Anti-Ganglioside Antibody Development
- Anti-Globoside Antibody Development
References:
- Hu, Xi, et al. "Towards the antiviral agents and nanotechnology-enabled approaches against parvovirus B19." Frontiers in Cellular and Infection Microbiology 12 (2022): 916012. https://doi.org/10.3389/fcimb.2022.916012
- Bieri, Jan, et al. "Globoside Is an Essential Intracellular Factor Required for Parvovirus B19 Endosomal Escape." Cells 13.15 (2024): 1254. https://doi.org/10.3390/cells13151254
- Distributed under Open Access license CC BY 4.0, without modification.
Supports
- Glycolipid
- GM3 Antibody in Cancer Immunotherapy
- GM3 Ganglioside in Disease & Anti-GM3 Antibody Tools
- Engineering High-Affinity scFv for Next-Generation GD2-CAR-T Therapy
- A Simple Guide to CANOMAD Syndrome
- Anti-Glycolipid Antibody Overview
- GM3 Ganglioside Overview
- Sulfatide and Anti-Sulfatide Antibodies Overview
